
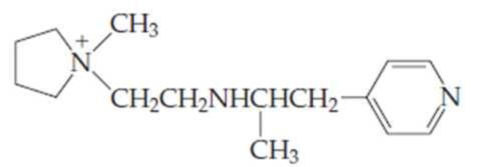
- (a) For the compound above, identify each nitrogen as either a primary, secondary, tertiary, quaternary, or aromatic
amine . - (b) Which amine group(s) would be able to provide a hydrogen bond? Which could accept a hydrogen bond?
(a)
Interpretation:
It should be identified that the each nitrogen in the given compound either as a primary, secondary, tertiary amine, or aromatic amine.
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
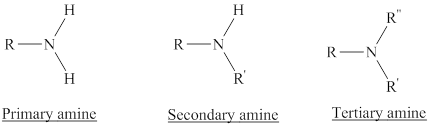
In a quaternary ammonium ion a nitrogen atom with four attached groups is positively charged. And their compounds are known as quaternary ammonium salt.
Example: Tetramethylammonium ion
Aniline is an aromatic amine compound and its structure is,
Answer to Problem 16.23UKC
Explanation of Solution
Structure of the given compound is,
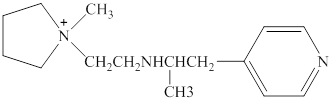
As per the concepts above mentioned,
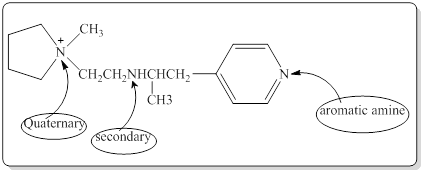
Each nitrogen atoms in the given compound can be identified either as a primary, secondary, tertiary amine or aromatic amine as follows,
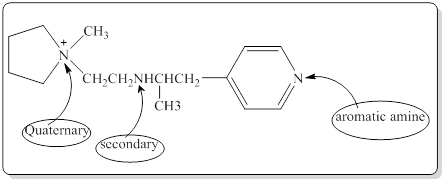
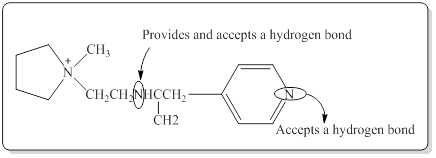
(b)
Interpretation:
The amine group which would be able to provide a hydrogen bond and accept a hydrogen bond in the given compound has to be identified.
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

Hydrogen bond is an attractive force established between hydrogen atom attached to a highly electronegative element and another highly electronegative element of the same or different molecule.
Answer to Problem 16.23UKC
Explanation of Solution
Structure of the given compound is,
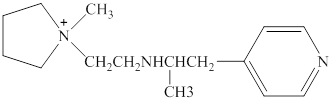
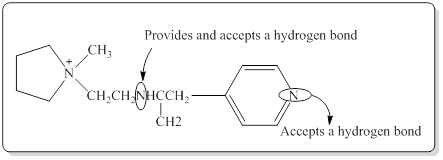
In a hydrogen bond strong partial positive charge on hydrogen attracts lone electron pair on oxygen or nitrogen.
Here in the given molecule, an amine group which was able to provide a hydrogen bond and accept a hydrogen bond and those amines can be identified as follows,
Want to see more full solutions like this?
Chapter 16 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
- Determine Km and Vmax from the michaelis menten grapharrow_forwardDetermine the Km and Vmax from the lineweuver burk grapharrow_forwardDo schwann cells produce or act as myelin in the peripheral nervous system? I know that they encase and wrap around axons, but where does the myelin come into play?arrow_forward
- The enzyme lactate dehydrogenase (LDH) catalyzes the conversion of pyruvate to lactatein skeletal muscle cells using NAD/NADH during anaerobic “balanced” fermentation.Answer the following questions about this reaction. (a) Write out the two reductive half reactions and indicate the E ̊' for each half reaction. Write out the full balanced reaction for the pyruvate to lactate rxn and indicate the ∆E ̊' for the reaction. (b) What is the free energy change under standard state conditions for thisreaction? Which direction is spontaneous?(c) Assume that in skeletal muscle cells the ratio of [NAD+] to [NADH] is 100, and that the[pyruvate] = 0.40 mM and [lactate] = 4.0 mM. What is the free energy change (∆G')for the conversion of pyruvate to lactate? Indicate the direction in which the reactionis spontaneous under these cellular conditions.arrow_forwardWhy did the authors worry about the temperature-dependent solubility of the carriers in thebilayer? How did the authors determine whether the effect of freezing the lipid bilayer wasto decrease the solubility of the carriers (nonactin and valinomycin) or whether the effectwas to impair their ability to diffuse through the membrane (decrease their mobility)?arrow_forwardKranse et. al. measured the temperature dependence of conductance using membranescontaining the phospholipids glyceryl dipalmitate and glyceryl distearate. Describe themodifications in membrane content that you would employ to: (a) shift the temperature of the phase transition (b) make the ion conductance curve for valinomycin andnonactin more like that of gramicidinarrow_forward
- Obtain the sequence for the 5-HT receptor HTR1A and generate a hydropathy plot usingthe ExPASY tool ProtScale, the appropriate window, and the Kyte-Doolittle weightingalgorithm. How many transmembrane domains are present in this receptor? Attach yourhydropathy plot to your assignment.arrow_forwardCompare and contrast the structural features of the ion carrier valinomycin with those of achannel former like gramicidin. How does structural information help explain the mechanismby which these molecules conduct ions across membranes?arrow_forwardA typical integral membrane protein has a stretch (or stretches) of ~20 hydrophobic aminoacids that form an α-helix that spans the bilayer (as is found in membrane proteins such asglycophorin A and bacteriorhodopsin). Compare and contrast the molecular and structural features of gramicidin with a membrane-spanning α-helix. Explain how gramicidin can forman ion channel when a typical membrane-spanning α-helix cannot (eg, glycophorin A).arrow_forward
- The titration curve of alanine shows the ionization of two functional groups with pK values of 2.34 and 9.69, corresponding to the ionization of the carboxyl and the protonated amino groups, respectively. The titration of di-, tri-, and larger oligopeptides of alanine also shows the ionization of only two functional groups, although the experimental pK values are different. The table summarizes the trend in pK values. Amino acid or peptide Ala Ala-Ala pKj pk₂ 2.34 9.69 3.12 8.30 Ala-Ala-Ala 3.39 8.03 Ala-(Ala)-Ala, n ≥ 4 3.42 7.94 Modify the molecules to show the oligopeptide Ala-Ala-Ala. You can modify the molecules by moving, adding, deleting, or changing atoms, bonds, or charges. C Select c Draw Templates More H с N 0 S Cl H H | | || H CH3 H CH, H CH₂ Complete the statements about the the pK, values of the Ala-Ala-Ala oligopeptide. The pK₁ value of 3.39 is associated with the -COO group of Ala-Ala-Ala. The pK2 value of 8.03 is associated with the -NH group of Ala-Ala-Ala. Erase Q2 Q…arrow_forwardFacts from the bacterium mals and to dept kan apa in a peptide with antidic properties. This peptide complex with the call membrance of other hacterial species, leading in bacterial death The structure of the peptide has been determined from (a) Cmplete acid hydes of the peptide, followed by amino acid analys, yielded quiar anunt of Lan, Om, Pfx, Prxa, and Wall Cmtiti, an amino acid od prosentin pockets but present in some peptides. Com has the tracture H *H,N-CH-CH-CH, -C- COO (b) The weight of the peptide in approximately 1,200 Th (c) The peptide failed to undergo hydrolysis when treated with the Hydrolysis of the carbonyl-terminal residue of a polypeptide une "NH, the year. This call there Pro or the police does not contain a froz (d) Treatment of the peptide with 1-haw-2,4-dicherer (11N1), followed by complete hydrolysis and ched only from and the derivative NO, Н ON NHCHI CH, CH, C coo +NH, (Hint: The 2,4-diphenyl derivative involves the amino group of a side chain rather than the…arrow_forwardElectrophoresis Macmillan Learning Chymotrypsin is a protease with a molecular mass of 25.6 kDa. The figure shows a stained SDS polyacrylamide gel with a single band in lane I and three bands of lower molecular weight in lane 2. Lane I contains a preparation of chymotrypsin and lane 2 contains chymotrypsin pre-treated with performic acid. 1 2 Why does performic acid treatment of chymotrypsin generate three bands in lane 2? ° Chymotrypsin self-digests on the carboxyl-terminal side of phenylalanine, tryptophan, or tyrosine residues. The three peptides are impurities in the original chymotrypsin sample. Performic acid cleaves proteins on the carboxyl-terminal side of lysine and arginine residues. Performic acid cleaves the disulfide bonds holding together the three subunits of chymotrypsin. Correct Answerarrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning





