
Concept explainers
(a)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the
Answer to Problem 16.32P
The name of the compound is 3,3-dimethylbutanal.
Explanation of Solution
The given molecular formula of the aldehyde is:
Here the numbering of the carbon chain will start from the right where -CHO group is present. It can be clearly seen that the longest carbon chain containing the aldehyde group consists of four carbon atoms and two methyl substituentsare attached to carbon number 3.
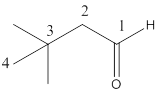
Therefore, the name of the compound becomes 3,3-dimethylbutanal.
(b)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon number 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Answer to Problem 16.32P
The name of the compound is 4-ethylheaxanal.
Explanation of Solution
The given molecular formula of the aldehyde is:
Here the numbering of the carbon chain will start from the right where -CHO group is present. It can be clearly seen that the longest carbon chain containing aldehydic group consists of six carbon atoms and an ethyl substituent is attached to carbon number4.
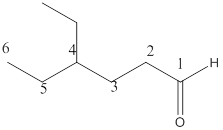
Therefore, the name of the compound becomes 4-ethylhexanal.
(c)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon no. 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Answer to Problem 16.32P
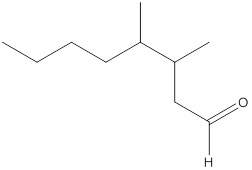
The name of the compound is 3,4-dimethyloctanal.
Explanation of Solution
The given molecular formula of the aldehyde is:
Here, the numbering of the carbon chain will start from the right where -CHO group is present. It can be clearly seen that the longest carbon chain containing the aldehyde group consists of eight carbon atoms and a methyl substituent is attached to carbon number 3 as well as carbon number 4.
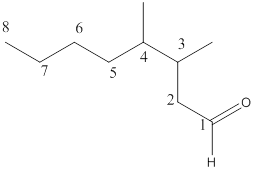
Therefore, the name of the compound becomes 3,4-dimethyloctanal.
(d)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon number 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Answer to Problem 16.32P
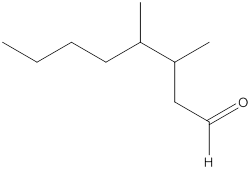
The name of the compound is 3-butylheptanal.
Explanation of Solution
The given molecular formula of the aldehyde can be written as follows:
Here, the numbering of the carbon chain will start from the right where -CHO group is present. It can be clearly seen that the longest carbon chain containing the aldehyde group consists of seven carbon atoms and abutyl substituent is attached to carbon number 3.
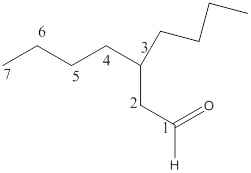
Therefore, the name of the compound becomes 3-butylheptanal.
(e)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon no. 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Answer to Problem 16.32P
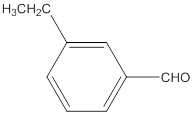
The name of the compound is 3-ethylbenzaldehyde.
Explanation of Solution
The given molecular formula of the aldehyde is:
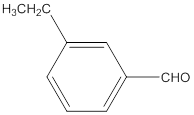
The name of the compound will be 3-ethylbenzaldehyde as the ethyl group is attached to the carbon number 3 of the parent molecule which is benzaldehyde. The benzene ring when attached to -CHO group is known as benzaldehyde.
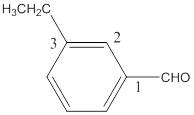
The naming of the molecule will start from the carbon to which aldehydic group is attached.
Want to see more full solutions like this?
Chapter 16 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- Please predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forward
- If we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forwarddrawing, no aiarrow_forwardIf CH3COCH2CH(OCH3)2 (4,4-dimethoxy-2-butanone) and hydrazine react, two isomeric products are formed. State their structure and which will be the majority.arrow_forward
- + Reset Provide the correct IUPAC name for the compound shown here. 4-methylhept-2-ene (Z)- (E)- 1-6-5-2-3-4- cyclo iso tert- sec- di tri hept hex oct meth eth pent ane yne ene ylarrow_forward+ Provide the correct IUPAC name for the compound shown here. Reset H3C H H C CH3 CH-CH3 1-3-methylpent ene trans- cis- 5-6-3-1-2-4- tert- tri sec- di cyclo iso but pent hex meth prop eth yl ane ene yne ☑arrow_forwarddrawing, no aiarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning 
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





