
Concept explainers
(a)
Interpretation:
Whether erythrulose contains an aldehyde or ketone should be identified.
Concept Introduction:
Compounds which contain carbonyl carbon bonded to hydrogen or carbon atom will be considered as
Aldehydes − at least one H atom bonded to the carbonyl group.
Ketones − has two alkyl groups bonded to the carbonyl group.
Answer to Problem 16.55P
Erythrulose contains a ketone group.
Explanation of Solution
Given:
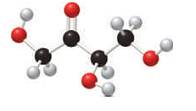
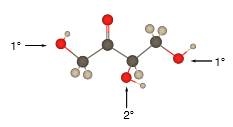
In the stick and ball structure, C atoms are representing in black color, H atoms in white color and O atoms in red color.
Stick and ball structure can be simplified as below;
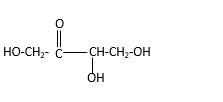
In erythrulose there is a carbonyl group that connects to two alkyl groups, thus, the erythrulose contains ketone.
(b)
Interpretation:
Hydroxyl groups in erythrulose should be classified as
Concept Introduction:
Alcohol- is an organic compound contains the group of
Answer to Problem 16.55P
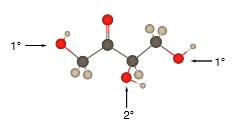
Explanation of Solution
In the below stick and ball structure, C atoms are representing in black color, H atoms in white color and O atoms in red color.
Stick and ball structure can be simplified as below;
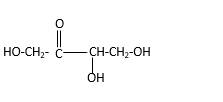
Bonds that are indicated in red color are the
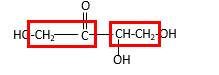
Bond that is indicated in blue color is the one that binds to two carbon atoms which makes an
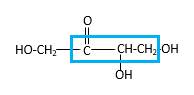
Therefore, OH groups in erythrulose can be assigned as below.
(c)
Interpretation:
The products should be identified by the reaction of erythrulose with Tollens reagent.
Concept Introduction:
Silver oxide in aqueous ammonium hydroxide reagent (
Answer to Problem 16.55P
No reaction.
Explanation of Solution
Addition of an O atom into a molecule is known as oxidation. If a carbonyl atom consists of a hydrogen atom directly connected to the carbonyl C, it will be oxidized in the presence of an oxidizing agent such as

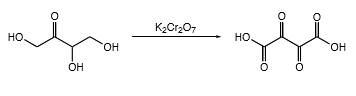
(d)
Interpretation:
The products should be identified by the reaction of erythrulose with
Concept Introduction:
Addition of an O atom into a molecule is known as oxidation. If a carbonyl atom consists of a hydrogen atom directly connected to the carbonyl C, it will be oxidized in the presence of an oxidizing agent such as
But, OH groups will be oxidized into respective carboxylic acids, aldehydes or ketones.
Answer to Problem 16.55P
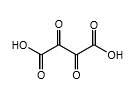
Explanation of Solution
In erythrulose molecule there are two primary alcohol groups and those groups will end up with a carboxylic acid and one secondary alcohol group in the erythrulose molecule will end up with a ketone by oxidation with
Want to see more full solutions like this?
Chapter 16 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- Q7: For the following reactions, indicate the reaction conditions that would provide the indicated product in a high yield. Note the major reaction pathway that would take place (SN1, SN2, E1, or E2) Note: There may be other products that are not shown. There maybe more than one plausible pathway. Br H3C OH H3C CI ... H3C SCH2CH3 CI i SCH2CH3 ཨ་ Br System Settarrow_forwardQ2: Rank the compounds in each of the following groups in order of decreasing rate of solvolysis in aqueous acetone. OSO2CF3 OSO2CH3 OH a. b. CI Brarrow_forwardох 4-tert-butyl oxy cyclohex-1-ene Incorrect, 1 attempt remaining The systematic name of this compound classifies the -OR group as a substituent of the hydrocarbon, which is considered the principal functional group. The ether substituent is named with the suffix 'oxy'. The general format for the systematic name of a hydrocarbon is: [prefix/substituent] + [parent] + [functional group suffix] Substituents are listed in alphabetical order. Molecules with a chiral center will indicate the absolute configuration at the beginning of its name with the R and S notation.arrow_forward
- 5. Compressibility (6 points total). The isothermal compressibility is a measure of how hard/easy it is to compress an object (how squishy is it?) at constant temperature. It is др defined as Br=-()=-(200²)T' (a) You might wonder why there is a negative sign in this formula. What does it mean when this quantity is positive and what does it mean when this quantity is negative? (b) Derive the formula for the isothermal compressibility of an ideal gas (it is very simple!) (c) Explain under what conditions for the ideal gas the compressibility is higher or lower, and why that makes sense.arrow_forward19. (3 pts) in Chapter 7 we will see a reaction of halocyclohexanes that requires that the halogen occupy an axial position with this in mind, would you expect cis-1-bromo-3-methylcyclohexane or trans-1-bromo-3-methylcyclohexane to be more reactive in this reaction? Briefly explain your choice using structures to support your answer. Mere-eries-cecleone) The tran-i-browse-3-methylcyclohexionearrow_forwardPlease help me calculate the undiluted samples ppm concentration. My calculations were 280.11 ppm. Please see if I did my math correctly using the following standard curve. Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EVSJL_W0qrxMkUjK2J3xMUEBHDu0UM1vPKQ-bc9HTcYXDQ?e=hVuPC4arrow_forward
- Provide an IUPAC name for each of the compounds shown. (Specify (E)/(Z) stereochemistry, if relevant, for straight chain alkenes only. Pay attention to commas, dashes, etc.) H₁₂C C(CH3)3 C=C H3C CH3 CH3CH2CH CI CH3 Submit Answer Retry Entire Group 2 more group attempts remaining Previous Nextarrow_forwardArrange the following compounds / ions in increasing nucleophilicity (least to most nucleophilic) CH3NH2 CH3C=C: CH3COO 1 2 3 5 Multiple Choice 1 point 1, 2, 3 2, 1, 3 3, 1, 2 2, 3, 1 The other answers are not correct 0000arrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the cured electron-pushing arrows for thw following reaction or mechanistic steps. be sure to account for all bond-breaking and bond making stepsarrow_forward
- Using the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.arrow_forwardSynthesis of Dibenzalacetone [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. Question 1 1 pt Question 2 1 pt Question 3 1 pt H Question 4 1 pt Question 5 1 pt Question 6 1 pt Question 7 1pt Question 8 1 pt Progress: 7/8 items Que Feb 24 at You do not have to consider stereochemistry. . Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ⚫ Separate multiple reactants using the + sign from the drop-down menu. ? 4arrow_forwardShown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


