
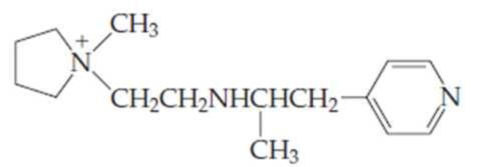
- (a) For the compound above, identify each nitrogen as either a primary, secondary, tertiary, quaternary, or aromatic
amine . - (b) Which amine group(s) would be able to provide a hydrogen bond? Which could accept a hydrogen bond?
(a)
Interpretation:
It should be identified that the each nitrogen in the given compound either as a primary, secondary, tertiary amine, or aromatic amine.
Concept introduction:
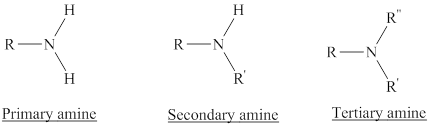
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
In a quaternary ammonium ion a nitrogen atom with four attached groups is positively charged. And their compounds are known as quaternary ammonium salt.
Example: Tetramethylammonium ion
Aniline is an aromatic amine compound and its structure is,
Answer to Problem 16.23UKC
Explanation of Solution
Structure of the given compound is,
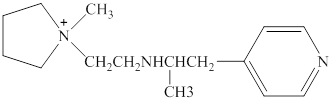
As per the concepts above mentioned,
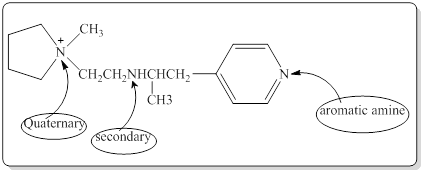
Each nitrogen atoms in the given compound can be identified either as a primary, secondary, tertiary amine or aromatic amine as follows,
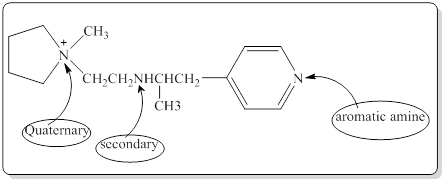
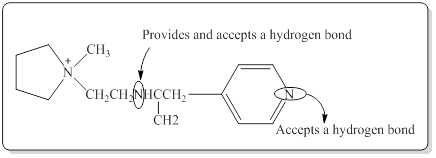
(b)
Interpretation:
The amine group which would be able to provide a hydrogen bond and accept a hydrogen bond in the given compound has to be identified.
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

Hydrogen bond is an attractive force established between hydrogen atom attached to a highly electronegative element and another highly electronegative element of the same or different molecule.
Answer to Problem 16.23UKC
Explanation of Solution
Structure of the given compound is,
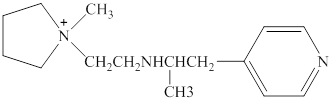
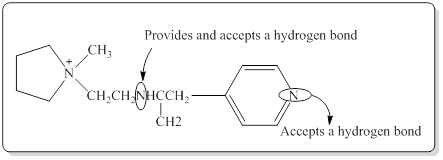
In a hydrogen bond strong partial positive charge on hydrogen attracts lone electron pair on oxygen or nitrogen.
Here in the given molecule, an amine group which was able to provide a hydrogen bond and accept a hydrogen bond and those amines can be identified as follows,
Want to see more full solutions like this?
Chapter 16 Solutions
Pearson eText Fundamentals of General, Organic, and Biological Chemistry -- Instant Access (Pearson+)
- 14. What is the IUPAC name of this compound? A) 6-hydroxy-4-oxohexanenitrile B) 5-cyano-3-oxo-1-pentanol C) 5-cyano-1-hydroxy-3-pentanone D) 1-cyano-5-hydroxy-3-pentanone E) 5-hydroxy-3-oxopentanenitrile HO. CNarrow_forward13. What is the IUPAC name of this compound? A) 5-hydroxy-3,3-dimethylpentanoic acid B) 3,3-dimethylpentanoic acid C) 3,3-dimethyl-1-oxo-1,5-pentanediol D) 1,5-dihydroxy-3,3-dimethylpentanal E) 4-hydroxy-2,2-dimethylbutanoic acid HO OHarrow_forwardHelp me understand how carbon disulfide leads to toxicity in the brain, using terms like distal axonopathy, neurofilaments, covalent cross-linking, adducts, etc.,...please intuitively explain what is happening and where and the effects of it. For example, I know that CS2 reacts with amide and sulfhydryl groups on proteins, but what proteins exactly and where are they located?arrow_forward
- What is the standard free energy change (in kJ/mole) of the spontaneous reaction between Oxygen and NADH to form H2O2 and NAD+?arrow_forwardRedox Chemistry: Give standard free energy changes expected for the following reactions:-Succinate -> fumarate (using FAD/FADH2)-Oxaloacetate -> Malate (using NAD/NADH)-NADH --> NAD+ (using FMN/FMNH2)-CoQ --> CoQH2 (using Cytochrome C)arrow_forwardGive examples of balanced redox reactions that match the following:-Catabolic-Anabolic-Oxidative-Reductivearrow_forward
- If there are 20uM of a GLUT2 transporter on the surface of a cell, each able to move 8 per second, and 50mM glucose outside of the cell, what is the flux into the cell in mM/sec?arrow_forwardA transporter is responsible for antiporting calcium and glucose. The transporter brings glucose into the cell and sends calcium out of the cell. If blood [calcium] = 2.55mM and intracellular [calcium] = 7uM, blood [glucose] = 5.2mM, and intracellular [glucose] = 40uM, what is the free energy of transport? Assume a membrane potential of 62mV (negative inside).arrow_forwardAn ATP-coupled transporter is used to import 1 phosphate from the extracellular environment. Intracellular phosphate exists at 65mM, while it is 2mM outside.Assume a free energy change of ATP hydrolysis of -42.7 kJ/mol. What is the net free energy change of the coupled reaction? Assume a membrane potential of 70mV.arrow_forward
- Another transporter brings 3 chloride ions into the cell. Outside, chloride has a concentration of 107mM, and 4mM inside the cell. Assuming a membrane potential of 62mV (negative inside), what is the free energy of transport of these ions?arrow_forwardFor the Oxaloacetate -> Malate reaction, assume the normal ratio of NAD/NADH, what is the maximum ratio of Malate/Oxaloacetate that will allow reaction progress?arrow_forwardA particular particle is trying to cross a membrane by simple diffusion from a high concentration of 20mM to a low concentration of 20uM. If a membrane is 15uM in width, and the diffusion coefficient of the particle is 5 uM/sec, what is the influx in uM/sec?arrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning





