
(a)
Interpretation:
The structure of thioester formed when reaction takes place between
Concept Introduction:
Thioesters are prepared by condensation of carboxylic acid with a thiol. A molecule of water is lost on this reaction. The reaction that takes place in producing thioesters is known as thioesterification reaction.
Thioesterification reaction is the one in which the carboxylic acid is condensed with a thiol in presence of strong acid catalyst to produce thioester. The general reaction scheme can be given as,
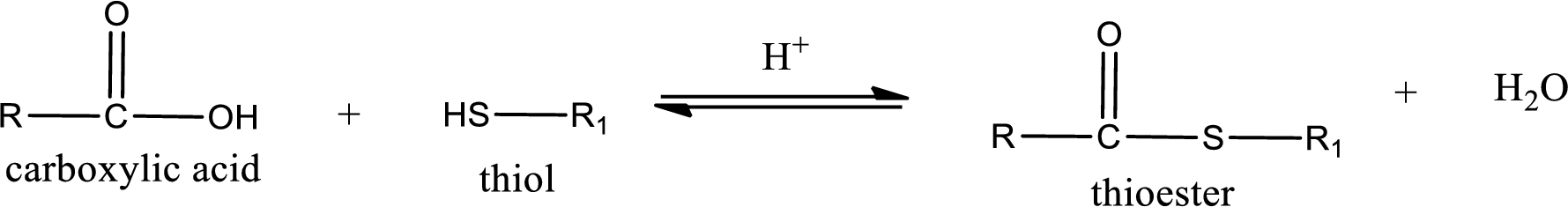
(b)
Interpretation:
The structure of thioester formed when reaction takes place between carboxylic acid given and the thiol given has to be drawn.
Concept Introduction:
Thioesters are prepared by condensation of carboxylic acid with a thiol. A molecule of water is lost on this reaction. The reaction that takes place in producing thioesters is known as thioesterification reaction.
Thioesterification reaction is the one in which the carboxylic acid is condensed with a thiol in presence of strong acid catalyst to produce thioester. The general reaction scheme can be given as,
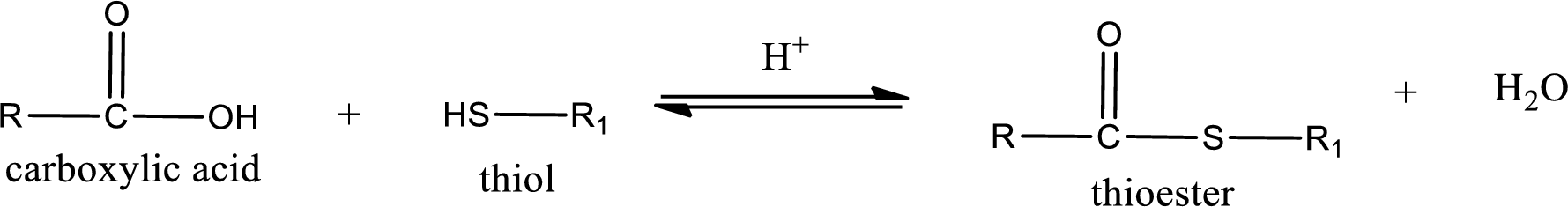
(c)
Interpretation:
The structure of thioester formed when reaction takes place between carboxylic acid given and the thiol given has to be drawn.
Concept Introduction:
Thioesters are prepared by condensation of carboxylic acid with a thiol. A molecule of water is lost on this reaction. The reaction that takes place in producing thioesters is known as thioesterification reaction.
Thioesterification reaction is the one in which the carboxylic acid is condensed with a thiol in presence of strong acid catalyst to produce thioester. The general reaction scheme can be given as,
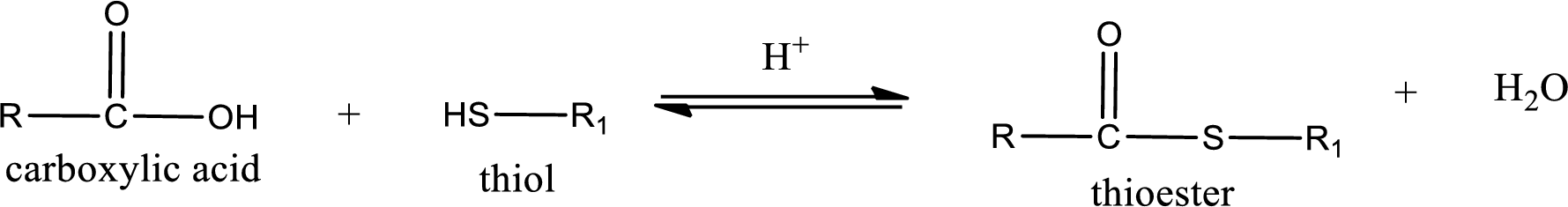
(d)
Interpretation:
The structure of thioester formed when reaction takes place between carboxylic acid given and the thiol given has to be drawn.
Concept Introduction:
Thioesters are prepared by condensation of carboxylic acid with a thiol. A molecule of water is lost on this reaction. The reaction that takes place in producing thioesters is known as thioesterification reaction.
Thioesterification reaction is the one in which the carboxylic acid is condensed with a thiol in presence of strong acid catalyst to produce thioester. The general reaction scheme can be given as,
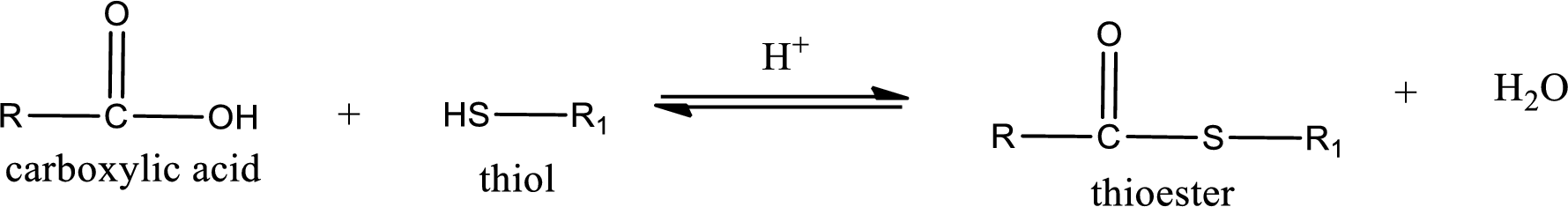
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- please fill in missing parts , thank youarrow_forwardplease draw in the answers, thank youarrow_forwarda. On this first grid, assume that the DNA and RNA templates are read left to right. DNA DNA mRNA codon tRNA anticodon polypeptide _strand strand C с A T G A U G C A TRP b. Now do this AGAIN assuming that the DNA and RNA templates are read right to left. DNA DNA strand strand C mRNA codon tRNA anticodon polypeptide 0 A T G A U G с A TRParrow_forward
- Please identify the curve shown below. What does this curve represent? Please identify A, B, C, D, and E (the orange oval). What is occurring in these regions?arrow_forwardPlease identify the test shown here. 1) What is the test? 2) What does the test indicate? How is it performed? What is CX? 3) Why might the test be performed in a clinical setting? GEN CZ CX CPZ PTZ CACarrow_forwardDetermine how much ATP would a cell produce when using fermentation of a 50 mM glucose solution?arrow_forward
- Determine how much ATP would a cell produce when using aerobic respiration of a 7 mM glucose solution?arrow_forwardDetermine how much ATP would a cell produce when using aerobic respiration to degrade one small protein molecule into 12 molecules of malic acid, how many ATP would that cell make? Malic acid is an intermediate in the Krebs cycle. Assume there is no other carbon source and no acetyl-CoA.arrow_forwardIdentify each of the major endocrine glandsarrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning





