
Organic Chemistry, 12e Study Guide/Student Solutions Manual
12th Edition
ISBN: 9781119077329
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 10PP
Practice Problem 16.10
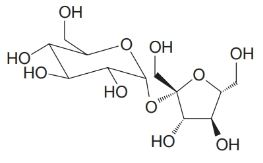
Shown below is the structural formula for sucrose (table sugar). Sucrose has two acetal groupings. Identify these.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
110. Compare the pressures given by (a) the ideal gas law, (b) the van der Waals equation, and
(c) the Redlic-Kwong equation for propane at 400 K and p = 10.62 mol dm³. The van der
Waals parameters for propane are a = 9.3919 dm6 bar mol-2 and b = 0.090494 dm³ mol−1.
The Redlich-Kwong parameters are A = 183.02 dm bar mol-2 and B =
0.062723 dm³ mol-1. The experimental value is 400 bar.
Research in surface science is carried out using stainless steel ultra-high vacuum chambers with pressures as low as 10-12 torr. How many molecules are there in a 1.00 cm3 volume at this pressure and at a temperature of 300 K? For comparison, calculate the number of molecules in a 1.00 cm3 volume at atmospheric pressure and room temperature. In outer space the pressure is approximately 1.3 x 10-11 Pa and the temperature is approximately 2.7 K (determined using the blackbody radiation of the universe). How many molecules would you expect find in 1.00 cm3 of outer space?
Draw the predominant form of
arginine at pH = 7.9. The pKa of the
side chain is 12.5. Include proper
stereochemistry.
H2N
OH
NH
H₂N
'N'
છ
H
pH = 7.9
Select to Draw
Chapter 16 Solutions
Organic Chemistry, 12e Study Guide/Student Solutions Manual
Ch. 16 - PRACTICE PROBLEM 16.1 (a) Give IUPAC substitutive...Ch. 16 - Prob. 2PPCh. 16 - Prob. 3PPCh. 16 - Practice Problem 16.4
Provide the reagents and...Ch. 16 - Prob. 5PPCh. 16 - Prob. 6PPCh. 16 - Prob. 7PPCh. 16 - Prob. 8PPCh. 16 - Prob. 9PPCh. 16 - Practice Problem 16.10
Shown below is the...
Ch. 16 - Prob. 11PPCh. 16 - Practice Problem 16.12
What product would be...Ch. 16 - Prob. 13PPCh. 16 - Practice Problem 16.14
Dihydropyran reacts readily...Ch. 16 - Practice Problem 16.15 Show how you might use...Ch. 16 - Practice Problem 16.16 (a) Show how you might...Ch. 16 - Practice Problem 16.17
In addition to...Ch. 16 - Practice Problem 16.18
Triphenylphosphine can be...Ch. 16 - Prob. 19PPCh. 16 - PRACTICE PROBLEM 16.20
Give the structure of the...Ch. 16 - PRACTICE PROBLEM 16.21 What would be the major...Ch. 16 - Prob. 22PCh. 16 - 16.23 Write structural formulas for the products...Ch. 16 - Give structural formulas for the products formed...Ch. 16 - 16.25 What products would be obtained when...Ch. 16 - Predict the major organic product from each of the...Ch. 16 - 16.27 Predict the major product from each of the...Ch. 16 - 16.28 Predict the major product from each of the...Ch. 16 - Prob. 29PCh. 16 - 16.30 Write detailed mechanisms for each of the...Ch. 16 - Prob. 31PCh. 16 - Prob. 32PCh. 16 - Show how you would convert benzaldehyde into each...Ch. 16 - 16.34 Show how ethyl phenyl ketone could be...Ch. 16 - Show how benzaldehyde could be synthesized from...Ch. 16 - Give structures for compounds AE. Cyclohexanol...Ch. 16 - Prob. 37PCh. 16 - Prob. 38PCh. 16 - Prob. 39PCh. 16 - Prob. 40PCh. 16 - Prob. 41PCh. 16 - Prob. 42PCh. 16 - 16.43 The structure of the sex pheromone...Ch. 16 - Provide reagents that would accomplish each of the...Ch. 16 - Write a detailed mechanism for the following...Ch. 16 - Prob. 46PCh. 16 - Dutch elm disease is caused by a fungus...Ch. 16 - Prob. 48PCh. 16 - Compounds W and X are isomers; they have the...Ch. 16 - Compounds Y and Z are isomers with the molecular...Ch. 16 - Compound A (C9H18O) forms a phenylhydrazone, but...Ch. 16 - Compound B (C8H12O2) shows a strong carbonyl...Ch. 16 - Prob. 53PCh. 16 - Prob. 54PCh. 16 - Prob. 55PCh. 16 - (a) What would be the frequencies of the two...Ch. 16 - Prob. 57PCh. 16 - Prob. LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Are extensive areas of nimbostratus clouds and periods of prolonged precipitation most likely to occur along a ...
Applications and Investigations in Earth Science (9th Edition)
Approximate mass of an isotope of iron is to be calculated. Concept introduction: An atom consists of subatomic...
Living By Chemistry: First Edition Textbook
1. How many cervical, thoracic, lumbar, sacral, and coccygeal vertebrae are normally present in the vertebral ...
Human Anatomy & Physiology (2nd Edition)
Give a molecular orbital description for each of the following: a. 1,3-pentadiene b. 1,4-pentadiene c. 1,3,5-he...
Organic Chemistry (8th Edition)
FOCUS ON INFORMATION In Bateslan mimicry, a palatable species gains protection by mimicking an unpalatable one....
Campbell Biology in Focus (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't used hand raitingarrow_forward142. A mixture of H2(g) and N2(g) has a density of 0.216 g/liter at 300 K and 500 torr. What is the mole fraction composition of the mixture?arrow_forwardOne liter of N2(g) at 2.1 bar and two liters of Ar(g) at 3.4 bar are mixed in a 4.0 liter flask to form an ideal gas mixture. Calculate the value of the final pressure of the mixture if the initial and final temperature of the gases are the same. Repeat this calculation if the initial temperature of the N2(g) and Ar(g) are 304 K and 402 K, respectively, and the final temperature of the mixture is 377 K.arrow_forward
- 10 5 4. These four 'H NMR spectra were recorded from different isomers with molecular formula CsH,CIO. They all contain a carbonyl group. Determine the structure of the different isomers. 0 10 5 0 10 5 10 9 8 7 6 5 4 3. 1 0 9 10 10 66 9 0 10 9 10 5 1 8 7 6 5 3 2 -a 8 7 6 5 1 10 9 8 7 6 5 22 2 1 0 3 2 16 1 0 3 2 1 2 6 0arrow_forwardUse the expression below to ⚫ calculate its value and report it to the proper number of significant digits (you may need to round your answer). ⚫ calculate the % error (or % relative error or % inherent error) ⚫ calculate the absolute error. (20.54±0.02 × 0.254±0.003) / (3.21±0.05) = Value: % Error: Absolute error: ± | % (only 1 significant digit) (only 1 significant digit)arrow_forwardIn each case (more ductile, more brittle, more tough or resistant), indicate which parameter has a larger value. parameter Elastic limit Tensile strength more ductile Strain at break Strength Elastic modulus more fragile more tough or resistantarrow_forward
- Nonearrow_forwardWhat functional groups are present in this IRarrow_forwardIn each case (more ductile, more brittle, more tough or resistant), indicate which parameter has a larger value. parameter Elastic limit Tensile strength more ductile Strain at break Strength Elastic modulus more fragile more tough or resistantarrow_forward
- 4) A typical bottle of pop holds carbon dioxide at a pressure of 5 atm. What is the concentration of carbon dioxide in th solution? 5) A stream flowing over rocks and such is exposed to the atmosphere and well aerated. What would be the nitrogen concentration in the water at 25°C? (Air pressure is 1.000 bar.)arrow_forwardUse the expression below to ⚫ calculate its value and report it to the proper number of significant digits (you may need to round your answer). ⚫ calculate the % error (or % relative error or % inherent error) ⚫ calculate the absolute error. (30.078±0.003) - (20.174±0.001) + (9.813±0.005) = Value: % Error: absolute error: ± % (only 1 significant digit) (only 1 significant digit)arrow_forwardDon't used Ai solution and don't used hand raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY