
Liquid octane (C8H18) enters a steady-flow combustion chamber at 25°C and 1 atm at a rate of 0.25 kg/min. It is burned with 50 percent excess air that also enters at 25°C and 1 atm. After combustion, the products are allowed to cool to 25°C. Assuming complete combustion and that all the H2O in the products is in liquid form, determine (a) the heat transfer rate from the combustion chamber, (b) the entropy generation rate, and (c) the exergy destruction rate. Assume that T0 = 298 K and the products leave the combustion chamber at 1 atm pressure.
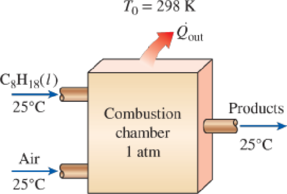
FIGURE P15–87
(a)
The rate of heat transfer from the combustion chamber.
Answer to Problem 83P
The rate of heat transfer from the combustion chamber is
Explanation of Solution
Write the energy balance equation using steady-flow equation.
Here, the total energy entering the system is
Substitute
Here, the enthalpy of formation for product is
Calculate the molar mass of the
Here, the number of carbon atoms is
Determine the rate of mole flow rates of the product.
Here, the mass flow rate is
Determine the heat transfer rate from the combustion chamber.
Conclusion:
Write the theoretical combustion equation of for
Here, liquid octane is
Calculate the stoichiometric coefficient of air by
Substitute
From the Table-26, “Enthalpy of formation, Gibbs function of formation, and absolute entropy at
| Substance | |
| -249,950 | |
| 0 | |
| 0 | |
| -285,830 | |
| -393,520 |
Refer Equation (VII), and write the number of moles of reactants.
Here, number of moles of reactant octane, oxygen and nitrogen is
Refer Equation (VII), and write the number of moles of products.
Here, number of moles of product carbon dioxide, water, oxygen and nitrogen is
Substitute the value table (I) of substance in Equation (II).
Therefore the heat transfer for
Substitute 8 for
Substitute
Substitute
Thus, the rate of heat transfer from the combustion chamber is
(b)
The entropy generation rate from the combustion chamber.
Answer to Problem 83P
The entropy generation rate from the combustion chamber is
Explanation of Solution
Write the expression for entropy generation during this process.
Write the combustion equation of Equation (VI)
Here, the entropy of the product is
Determine the entropy at the partial pressure of the components.
Here, the partial pressure is
Determine the entropy generation rate from the combustion chamber.
Conclusion:
Refer Equation (X) for reactant and product to calculation the entropy in tabular form as:
For reactant entropy,
| Substance |
(T, 1 atm) | ||||
| 1 | 1.00 | 360.79 | --- | 360.79 | |
| 18.75 | 0.21 | 205.14 | -12.98 | 4089.75 | |
| 70.50 | 0.79 | 191.61 | -1.96 | 13646.69 | |
For product entropy,
| Substance |
(T, 1 atm) | ||||
| 8 | 0.0944 | 213.80 | -19.62 | 1867.3 | |
| 9 | --- | 69.92 | --- | 629.3 | |
| 6.25 | 0.0737 | 205.04 | -21.68 | 1417.6 | |
| 70.50 | 0.8319 | 191.61 | -1.53 | 13616.3 | |
Substitute
Substitute
Thus, the entropy generation rate from the combustion chamber is
(c)
The exergy destruction rate from the combustion chamber.
Answer to Problem 83P
The exergy destruction rate from the combustion chamber is
Explanation of Solution
Write the expression for exergy destruction during this process.
Here, the thermodynamic temperature of the surrounding is
Conclusion:
Substitute
Thus, the exergy destruction rate from the combustion chamber is
Want to see more full solutions like this?
Chapter 15 Solutions
Thermodynamics: An Engineering Approach
- 4. The rod ABCD is made of an aluminum for which E = 70 GPa. For the loading shown, determine the deflection of (a) point B, (b) point D. 1.75 m Area = 800 mm² 100 kN B 1.25 m с Area = 500 mm² 75 kN 1.5 m D 50 kNarrow_forwardResearch and select different values for the R ratio from various engine models, then analyze how these changes affect instantaneous velocity and acceleration, presenting your findings visually using graphs.arrow_forwardQu. 7 The v -t graph of a car while travelling along a road is shown. Draw the s -t and a -t graphs for the motion. I need to draw a graph and I need to show all work step by step please do not get short cut from dtnaarrow_forward
- An unpressurized cylindrical tank with a 100-foot diameter holds a 40-foot column of water. What is total force acting against the bottom of the tank?arrow_forward7. In the following problems check to see if the set S is a vector subspace of the corresponding R. If it is not, explain why not. If it is, then find a basis and the dimension. (a) S = (b) S = {[],+,"} X1 x12x2 = x3 CR³ {[1], 4+4 = 1} CR³ X2arrow_forwardAAA Show laplace transform on 1; (+) to L (y(+)) : SY(s) = x (0) Y(s) = £ [lx (+)] = 5 x(+) · est de 2 -St L [ y (^) ] = So KG) et de D 2 D D AA Y(A) → Y(s) Ŷ (+) → s Y(s) -yarrow_forward
- 1) In each of the following scenarios, based on the plane of impact (shown with an (n, t)) and the motion of mass 1, draw the direction of motion of mass 2 after the impact. Note that in all scenarios, mass 2 is initially at rest. What can you say about the nature of the motion of mass 2 regardless of the scenario? m1 15 <+ m2 2) y "L χ m1 m2 m1 בז m2 Farrow_forward8. In the following check to see if the set S is a vector subspace of the corresponding Rn. If it is not, explain why not. If it is, then find a basis and the dimension. X1 (a) S = X2 {[2], n ≤ n } c X1 X2 CR² X1 (b) S X2 = X3 X4 x1 + x2 x3 = 0arrow_forward2) Suppose that two unequal masses m₁ and m₂ are moving with initial velocities V₁ and V₂, respectively. The masses hit each other and have a coefficient of restitution e. After the impact, mass 1 and 2 head to their respective gaps at angles a and ẞ, respectively. Derive expressions for each of the angles in terms of the initial velocities and the coefficient of restitution. m1 m2 8 m1 ↑ บา m2 ñ Вarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





