
Concept explainers
(a)
Interpretation:
Whether CH3(CH2)6CH3 is optically active or not needs to be determined.
Concept introduction:
The ability of substances to rotate the plane of polarized light passes through it is defined as an optical activity of the substance. The molecules with chiral center or asymmetric in nature can show optical activity. Thus, the presence of the chiral center in the molecule is important in this case.
(b)
Interpretation:
Whether the given molecule is optically active or not needs to be determined.
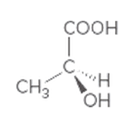
Concept introduction:
The ability of substances to rotate the plane of polarized light passes through it is defined as the optical activity of the substance. The molecules with chiral center or asymmetric in nature can show optical activity. Thus, the presence of the chiral center in the molecule is important in this case.
(c)
Interpretation:
Whether the given molecule is optically active or not needs to be determined.
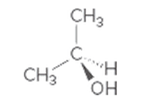
Concept introduction:
The ability of substances to rotate the plane of polarized light passes through it is defined as the optical activity of the substance. The molecules with chiral center or asymmetric in nature can show optical activity. Thus, the presence of the chiral center in the molecule is important in this case.
(d)
Interpretation:
Whether the given molecule is optically active or not needs to be determined.
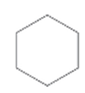
Concept introduction:
The ability of substances to rotate the plane of polarized light passes through it is defined as the optical activity of the substance. The molecules with chiral center or asymmetric in nature can show optical activity. Thus, the presence of the chiral center in the molecule is important in this case.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Connect One Semester Access Card for General, Organic, & Biological Chemistry
- Blocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forwardI dont understand this.arrow_forwardCan you please explain this prooblem to me, show me how the conjugation is added, did I add them in the correct places and if so please show me. Thanks!arrow_forward
- Basic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forward
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


