
Concept explainers
a)
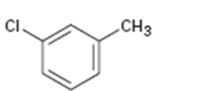
Interpretation:
Whether the compound given is ortho-, meta-, or para-disubstituted is to be stated.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta (m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2-relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring.
To state:
Whether the compound given is ortho-, meta-, or para-disubstituted.
b)
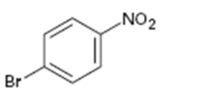
Interpretation:
Whether the compound given is ortho-, meta-, or para-disubstituted is to be stated.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta (m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2-relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring.
To state:
Whether the compound given is ortho-, meta-, or para-disubstituted.
c)
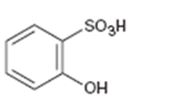
Interpretation:
Whether the compound given is ortho-, meta-, or para-disubstituted is to be stated.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta (m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2-relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring.
To state:
Whether the compound given is ortho-, meta-, or para-disubstituted.
Trending nowThis is a popular solution!

Chapter 15 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- Indicate the product that is obtained if the benzotriazol reacts with dimethyl sulfate.arrow_forwardIndicate how to obtain 2-metilbencimidazol from 1,2-diaminobenzene.arrow_forwardbreak down both reactions shown and explain it correctly using the bromonium ion mechanism, instead of the (disproven) carbocation-based mechanism.arrow_forward
- Indicate how from 1,2-diaminobenzene to obtain 1-metilbenzotriazol.arrow_forward-C = C - C - + Br₂ + I" -> -C-C-c -C = C -C- + Br² + I₂ -C=C Br I + Brū + Iz -7- C - C-C- I Br Mechanism; - C = c - c - + Br - Br > - C-c-c- Br -C-C-C- + 1 - - -Ċ-Ċ'-c' - Br Br Iarrow_forwardWrite the mechanism of the esterification reaction (please show the mechanism included line pairs and arrows)arrow_forward
- How do I break down the reaction shown on the chalkboard and explain it correctly using the bromonium ion mechanism, instead of the (disproven) carbocation-based mechanismarrow_forward¿Qué the product is obtained from tetraethoxypropano and hidrazina?. Indicate the reason why the corresponding dial is used.arrow_forwardIf CH3COCH2CH(OCH3)2 is reacted with hydrazine, two isomeric products are formed. Indicate their structures and the major product.arrow_forward
- Is it possible to obtain addition derivatives to nitrogen in position 2 of pyrazoles by reaction with electrophilic agents? Reason for this.arrow_forwardStarting from 1,3-dicarbonyl derivatives to obtain isooxazoles and isothiazoles. Indicate whether synthetic methods exist.arrow_forwardIn the synthesis of benzotriazole, adding NaNO2 heats the solution. State the reason.arrow_forward

 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





