
ND STONY BROOK UNIVERSITY LOOSELEAF GENETICS: FROM GENES TO GENOMES
6th Edition
ISBN: 9781260406092
Author: HARTWELL, Leland, HOOD, Leroy, Goldberg, Michael
Publisher: Mcgraw-hill Education/stony Brook University
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 9P
An example of a gene-targeting DNA plasmid vector for insertion of a transgene into chloroplast DNA by biolistic transformation is shown in the following diagram. The plasmid DNA can be prepared in large quantities in E. coli before being shot into plant cells with a gene gun. Match the component of the construct with its function. (RE1, RE2, and RE3 are different restriction enzyme recognition sites unique to the plasmid vector.)
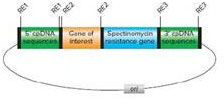
| a. spectinomycin | 1. DNA that mediates resistance gene integration |
| b. cpDNA sequences | 2. gene used to select chloroplast transformants |
| c. RE2 | 3. sequence for plasmid replication in E. coli |
| d. ori | 4. sites at which targeting chloroplast DNA can be inserted into the vector |
| e. RE1 and RE3 | 5. site at which transgene can be inserted into the vector |
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Biopharmaceutics and Pharmacokinetics:Two-Compartment Model Instant Absorption Questions
Calculate these :
a) B1,
b) B2,
c) hybrid rate constant (1)
d) hybrid rate constant (2)
e) t1/2,dist
f) t1/2,elim
g) k10
h) k12
i) k21
j) initial concentration (C0)
k) central compartment volume (V1)
l) steady-state volume (Vss)
m) clearance (CL) AUC (0→10 min) using trapezoidal rule
n) AUC (20→30 min) using trapezoidal rule
o) AUCtail (AUC360→∞)
p) total AUC (using short cut method)
q) volume from AUC (VAUC)
Use the Henderson-Hasselbalch equation for a propanoic acid solution (CH₂CH₂CO₂H, pK₁ = 4.874) to calculate the quotient
[A-]/[HA] at three different pH values.
pH = 4.479
[A-]
[HA]
[A-]
pH
= 4.874
[HA]
=
pH = 5.220
[A-]
=
[HA]
In order to establish the expiration date of perishable food, growth curve data must be collected. Once the microbial load is so high that it poses a hazard to human health, the food item is no longer considered safe (expired). Generally a load of x50,000
bacteria/gram is considered unsafe.
Your task is to determine the microbial growth curves for MicroYo, a new brand of yogurt. The growth is determined by sampling the yogurt and growing the bacterial isolates in broth culture which is then serially diluted by a total of x10,000 and inoculated onto
standard petri plates of nutrient agar. The following colony counts are measured:
Time (days)
MicroYo colony count#
1
1
4
1
12
2
16
20
4
7
What day should you recommend expiring the yogurt (the last possible date before the microbial load is unsafe).
12
4
20
16
Chapter 15 Solutions
ND STONY BROOK UNIVERSITY LOOSELEAF GENETICS: FROM GENES TO GENOMES
Ch. 15 - Match each numbered item with the most closely...Ch. 15 - Assuming human cells have on average 1000...Ch. 15 - Reverse translation is a term given to the process...Ch. 15 - The human nuclear genome encodes tRNAs with 32...Ch. 15 - The human mitochondrial genome includes no genes...Ch. 15 - How do you know if the halibut you purchased at...Ch. 15 - Is each of these statements true of chloroplast or...Ch. 15 - Suppose you are characterizing the DNA of a...Ch. 15 - An example of a gene-targeting DNA plasmid vector...Ch. 15 - Which of the following characteristics of...
Ch. 15 - The Saccharomyces cerevisiae nuclear gene ARG8...Ch. 15 - The so-called hypervariable regions HV1 and HV2 of...Ch. 15 - Suppose a new mutation arises in a mitochondrial...Ch. 15 - Describe at least two ways in which the...Ch. 15 - Why are severe mitochondrial or chloroplast gene...Ch. 15 - Suppose you are examining a newly found plant...Ch. 15 - A form of male sterility in corn is inherited...Ch. 15 - Plant breeders have long appreciated the...Ch. 15 - A mutant haploid strain of Saccharomyces...Ch. 15 - Prob. 20PCh. 15 - What characteristics in a human pedigree suggest a...Ch. 15 - The first person in the family represented by the...Ch. 15 - In 1988, neurologists in Australia reported the...Ch. 15 - If you were a genetic counselor and had a patient...Ch. 15 - Kearns-Sayre syndrome KSS, Pearson syndrome, and...Ch. 15 - Many clinically relevant mitochondrial diseases...Ch. 15 - Leigh syndrome is characterized by psychomotor...Ch. 15 - All mutations in mitochondrial genes ultimately...Ch. 15 - How could researchers have determined that the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- 9. Chicken combs in chickens is an example where you see interactions between genes. See potential genotypes and phenotypes below. Which genotype, when mated to a rose comb chicken, will produce progeny that are 50% walnut comb and 50% pea comb? walnut (RRPP) walnut (RrPP) pea (rrPP) walnut (RRPP) walnut (RrPp) pea (rrPp) rose rose single (RRPP) (Rrpp) (rrpp)arrow_forwardDescribe a compound light microscope and its importance in microbiology (2) examples of at least two microbes viewed under a compound light microscope and their general characteristics (note: the microbes you choose do not need to be the ones outlined in the above tutorial video) and (3) at least one source you used for the information included in your infographic.arrow_forwardPrice of visit Number of visits $700 0 $600 [1 $500 2 $400 3 $300 4 00000 The Table blow gives the demand curve for doctor visits for Elena. If the price of a doctor's visit is $600, and Elena does not have health insurance, she will visit the doctor times. If Elena obtains 50% coinsurance (the company pays 50% of the medical bill, Elena pays 50%), then Elena will visit the doctor times. 1; 2 0; 3 0; 2 1;4 2; 1arrow_forward
- P 200 150- 100 50 w/instrance/ w/insurance 2 100 Demand Assume that the white curve (labeled "Demand") represents an individual's true demand for this particular health care service. The coinsurance associated with insurance option 1 (in blue) is likely _. 0000 100% 25% 50% 0%arrow_forwardUse the figure below. Bob and Nancy have the same income and total utility.. willingness to pay for an insurance premium will be lower than because they are. risk- averse. Total utility Current utility Bob's utility Nancy's utility 0000 Bob; Nancy; less Nancy; Bob; less Nancy; Bob; more Bob; Nancy; more Current Income incomearrow_forwardConsider the figure below. Suppose the true price of a health care service is P1. Suppose further that the individual has obtained insurance that has a fixed copayment for this particular service. The copayment is represented by price P2. represents the quantity of the service the individual would consume without insurance. quantity of the service the individual would consume with the insurance. Health Care Service represents the P. P₂ a Q1;Q2 Q2; Q3 Q1; Q3 Q3; Q1 Q2; Q1 फ f Q ८ g d h Q3\D 7Q 00000arrow_forward
- The table shows the utility Jordan receives at various income levels, but they do not know what their income will be next year. There is a 15% chance their income will be $25,000, a 20% chance their income will be $35,000, and a 65% chance their income will be $45,000. We know that Jordan is Income $25,000 Utility 2,800 30,000 3,200 35,000 3,500 40,000 3,700 45,000 3,800 ☐ none of the above 0 000 risk taker (lover) because their marginal utility of income is increasing risk neutral because their marginal utility of income is constant risk averse because their marginal utility of income is decreasing risk neutral because their marginal utility of income is decreasingarrow_forwardOOOO a d+e d a+b+c Consider the figure below. Suppose the true price of a health care service is P1. Suppose further that the individual has obtained insurance that has a fixed copayment for this particular service. The copayment is represented by price P2. The social loss from moral hazard if the individual has copayment P2 is represented graphically by the area(s): Health Care Service P. a No 4 ८ e g Q2 Q3 Darrow_forwardOOO O The table shows the utility Jordan receives at various income levels, but they do not know what their income will be next year. There is a 15% chance their income will be $25,000, a 20% chance their income will be $35,000, and a 65% chance their income will be $45,000. We know that Jordan's expected income is. Their utility from their expected income is_ Income $25,000 Utility 2,800 30,000 3,200 35,000 3,500 40,000 3,700 45,000 3,800 $45,000; 3,800 $40,000; 3,700 $25,000; 2,800 $35,000; 3,500 $30,000; 3,200arrow_forward
- Question 1 Classify the Bird Mark 7; how is it: Powered Triggered Cycled Classify brid mark 7 Powered: By gas (oxygen) Triggered: Negative Pressure, caused by the patient’s inspiratory effort Cycled: The machine stops delivering gas and allows for exhalationarrow_forwardHypothetical "pedigree" for Sickle Cellarrow_forwardwould this be considered a novel protein and if not how can I fix it so it is and can you draw the corrections pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College
Molecular Techniques: Basic Concepts; Author: Dr. A's Clinical Lab Videos;https://www.youtube.com/watch?v=7HFHZy8h6z0;License: Standard Youtube License