
Interpretation:
The rate law should be calculated along with the value of rate constant for the given reaction.
Concept Introduction:
Rate law gives the relationship between the rate of the reaction and the reactant concentrations.
The general reaction is:
Rate of above reaction is expressed as:
Where, k = rate constant
The rate constant is defined as the proportionality constant which shows the relationship between concentration of reactants and rate of
Answer to Problem 27E
Rate law expression is:
The rate constant is equal to
Explanation of Solution
Given reaction:
The chemical reaction is:
The given data is:
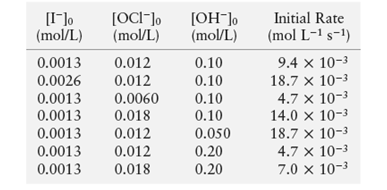
The given chemical reaction is:
The general rate law for above reaction is:
Here, concentration of hydroxide affect the rate, thus considered in rate law.
Where,
Now, put the values in above expression for first and second experiment.
Divide (1) and (2)
Hence, value of x = 1.
Now, put the values in above expression for first and third experiment.
Divide (1) and (3)
Hence, value of y = 1.
Now, put the values in above expression for first and fifth experiment.
Divide (1) and (4)
Hence, value of z=
Put the value of x, y and z in rate law expression:
The above expression is rearranged as:
Put the values in above expression from experiment 1 to determine the value of rate constant (k).
Thus, the rate constant is equal to
Want to see more full solutions like this?
Chapter 15 Solutions
Chemical Principles
- How many chiral centers/stereocenters are there in the following molecule? 1 2 3 4arrow_forwardWhich of these correspond to the molecule: 2,5-dimethylheptanearrow_forwardGiven the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forward
- Which one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forwardThe structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forwardWhich of the following compounds would you most appropriately call hydrophobic? ○ CH4 H2CO CO HCI ○ NaClarrow_forward
- Which of the following triglycerides would you most expect to be a liquid at room temperature? saturated fat trans monounsaturated fat trans polyunsaturated fat cis monounsaturated fat ○ cis polyunsaturated fatarrow_forwardWhich best describes the intermolecular forces present in NH3? dispersion forces only hydrogen bonding and dispersion forces dipole-dipole, hydrogen bonding, and dispersion forces dipole-dipole forces only ion-dipole and dispersion forcesarrow_forwardList three structural features and corresponding absorption ranges that can be used to identify cyclohexene by IR spectroarrow_forward
- The following chemical structure represents a molecule of what molecular formula? N.arrow_forwardPredict the product(s) of the following reactions. If no reaction, write "NR". a) b) HNO3 H2SO4 SO3 H2SO4 c) Bra FeBr3 Br2, FeBrз OCH3 d) تمنی e) HO f) SO3 H2SO4 CH3Cl NO2 AICI3arrow_forwardHow could you get from the starting material to product? A. OH B. OH Όarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





