
(a)
Interpretation:
The stoichiometry concept map is to be drawn and the mass of reacted methane
Concept introduction:
Stoichiometry measures the amount of reactant and product consumed or formed in a
Answer to Problem 23E
The stoichiometry concept map is shown below.
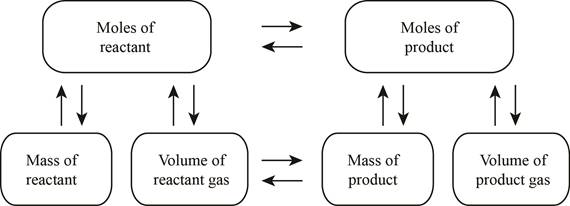
The mass of the reacted methane is
Explanation of Solution
It is given that
The stoichiometry concept map is shown below.
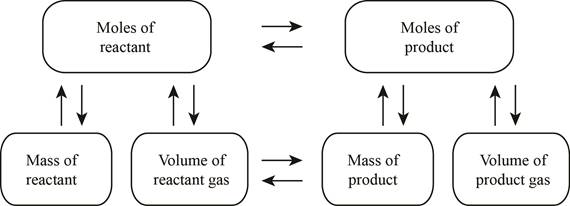
Figure 1
The chemical reaction (1) should be balanced first to calculate the mass of reacted methane.
The number of oxygen atoms is not equal on both sides. A coefficient of
The number of hydrogen atoms is not equal on both sides. A coefficient of
The balanced equation is shown below.
The mass of methane reacted is equal to the mass of
The molar mass of hydrogen is
The molar mass of oxygen is
Therefore, the number of moles of methane is calculated from the number of moles of water using a stoichiometric ratio as shown below.
For
The mass of methane reacted can be calculated from the number of moles of methane which is
The molar mass of carbon is
The molar mass of hydrogen is
The number of moles of methane can be calculated from the relation shown below.
The mass of methane for
Therefore, the mass of methane is
The stoichiometry concept map is shown in Figure 1. The mass of methane consumed in the reaction is
(b)
Interpretation:
The volume of carbon dioxide
Concept introduction:
Stoichiometry measures the amount of reactant and product consumed or formed in a chemical reaction. It gives the relationship between the reactants and products. Stoichiometry concept maps are used to determine the relation of reactant and product in terms of stoichiometry.
Answer to Problem 23E
The stoichiometry map is shown below.
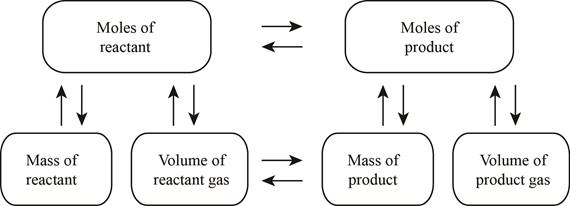
The volume of carbon dioxide produced at
Explanation of Solution
The stoichiometry concept mass is shown below.
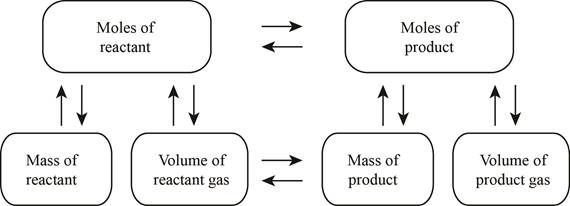
Figure 1
The balanced chemical reaction is stated in part (a) named as equation (2) is used to calculate the volume of
The equation (2) shows that 1 mole of methane is consumed to form 1mole of carbon dioxide.
Therefore, the volume for
Therefore, the volume of
The volume of
Want to see more full solutions like this?
Chapter 15 Solutions
INTRODUCTORY CHEMISTRY
- The following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forward
- How many chiral centers/stereocenters are there in the following molecule? 1 2 3 4arrow_forwardWhich of these correspond to the molecule: 2,5-dimethylheptanearrow_forwardGiven the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





