
(a)
Interpretation:
The stoichiometry concept map is to be drawn and the mass of
Concept introduction:
Stoichiometry measures the amount of reactant and product consumed or formed in a
Answer to Problem 26E
The stoichiometry concept map is shown below.
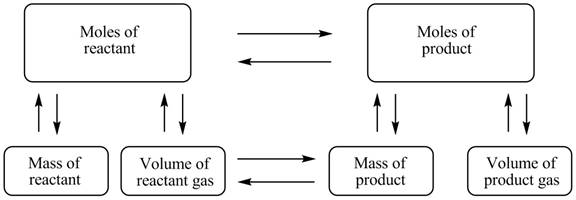
The mass of
Explanation of Solution
It is given that
The given chemical reaction is stated below.
The stoichiometry concept map is shown below.
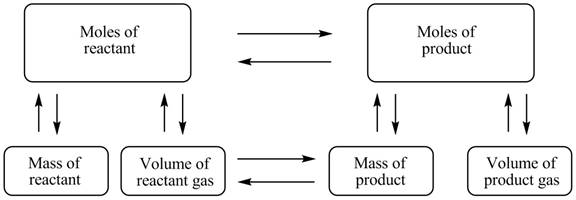
Figure 1
The chemical reaction (1) should be balanced first to calculate the mass of
The number of potassium atom and the hydrogen atom are not equal on both sides. A coefficient of
The balanced equation is stated below.
The equation (2) shows that
At
Therefore, the number of moles of
The molar mass of potassium is
The molar mass of oxygen is
The molar mass of hydrogen is
The molar mass of carbon is
The number of moles of
The mass for
Therefore, the mass of
The stoichiometry concept map is shown in Figure 1. The mass of
(b)
Interpretation:
The stoichiometry concept map is to be drawn and the mass of the
Concept introduction:
Stoichiometry measures the amount of reactant and product consumed or formed in a chemical reaction. It gives the relationship between the reactants and products. Stoichiometry concept maps are used to determine the relation of reactant and product in terms of stoichiometry.
Answer to Problem 26E
The stoichiometry map is shown below.
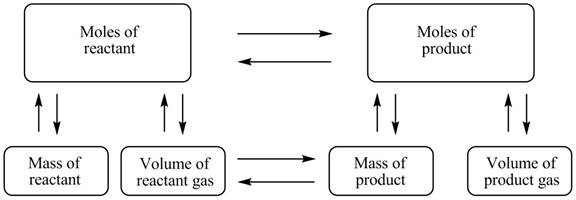
The mass of
Explanation of Solution
The stoichiometry concept mass is shown below.
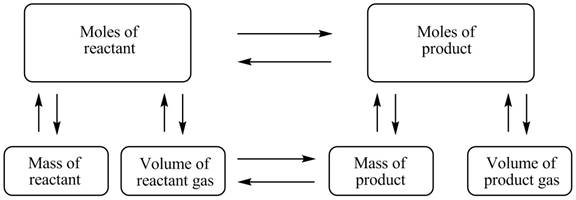
Figure 1
The number of potassium atom and the hydrogen atom are not equal on both sides. A coefficient of
The balanced equation is stated below.
The equation (2) shows that
The molar mass of
The mass calculated of
The number of moles of
The molar mass of potassium is
The molar mass of oxygen is
The molar mass of carbon is
The mass of
The number of moles of
The mass for
Therefore, the mass of
The mass of
Want to see more full solutions like this?
Chapter 15 Solutions
INTRODUCTORY CHEMISTRY
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardDraw a Newman projection for the molecule below from the perspective indicated. Which of the groups (letters A-H) are methyl groups? CH3 H H H A H B ☑ >> H. ABCDEFG I H -H CH3 G D CH F E Numeric 4 points How many gauche interactions exist in the conformation shown in the previous problem? 1arrow_forward
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward
- Pls help.arrow_forward13) When solid barium phosphate is in equilibrium with its ions, the ratio of barium ions to phosphate ions would be: a. 1:1 b. 2:3 c. 3:2 d. 2:1 14) The pH of a 0.05 M solution of HCl(aq) at 25°C is 15) The pH of a 0.20 M solution of KOH at 25°C isarrow_forwardPls help.arrow_forward
- Pls help.arrow_forward16) A 2.0 L flask containing 2.0 x 10-3 mol H2(g), 3.0 x 10-3 mol Cl2(g), and 4.0 x 10-3 mol HCl(g) at equilibrium. This system is represented by the following chemical equation: H2 (g) + Cl2 (g) → 2HCl(g) Calculate the equilibrium constant for this reaction.arrow_forward7) The pH of a 0.05M solution of HCl(aq) at 25°C is a. 1.3 b. 2.3 c. 3.3 d. 12.7arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





