
Concept explainers
(a)
Interpretation:
A concept map is to be drawn and the liters of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 17E
The concept map is shown below.
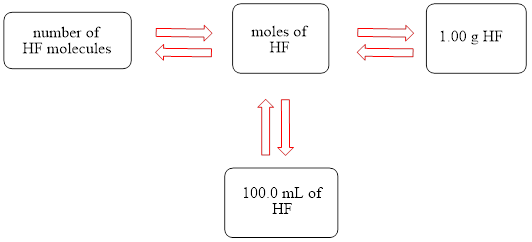
The liters of
Explanation of Solution
When

Figure 1
The formula to calculate the number of moles of
The mass of
The molar mass of
Substitute the mass and molar mass of
The volume occupied by
The formula to calculate the volume occupied by
Substitute the volume of
Therefore, the liters of
The liters of
(b)
Interpretation:
A concept map is to be drawn and the molecules of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 17E
The concept map is shown below.

The molecules of
Explanation of Solution
When

Figure 1
The formula to calculate the number of moles of
The mass of
The molar mass of
Substitute the mass and molar mass of
The molecules present in
The formula to calculate the molecules occupied by
Substitute the molecules in
Therefore, the molecules of
The molecules of
(c)
Interpretation:
A concept map is to be drawn and molar concentration of the hydrofluoric solution in the solution in when
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 17E
The concept map is shown below.

The molar concentration of the hydrofluoric acid solution is
Explanation of Solution
When

Figure 1
The formula to calculate the number of moles of
The mass of
The molar mass of
Substitute the mass and molar mass of
The number of moles in
The relation between
The probable unit factors are given below.
The unit factor to determine
Therefore, the volume in
The formula to determine molarity is shown below.
Where
•
•
•
Substitute the value of number of moles and volume in equation (1).
The relation between
The unit factors are given below.
The unit factor to determine
Therefore,
Therefore, the molar concentration of
The molar concentration of
Want to see more full solutions like this?
Chapter 15 Solutions
INTRODUCTORY CHEMISTRY
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardDraw a Newman projection for the molecule below from the perspective indicated. Which of the groups (letters A-H) are methyl groups? CH3 H H H A H B ☑ >> H. ABCDEFG I H -H CH3 G D CH F E Numeric 4 points How many gauche interactions exist in the conformation shown in the previous problem? 1arrow_forward
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward
- Pls help.arrow_forward13) When solid barium phosphate is in equilibrium with its ions, the ratio of barium ions to phosphate ions would be: a. 1:1 b. 2:3 c. 3:2 d. 2:1 14) The pH of a 0.05 M solution of HCl(aq) at 25°C is 15) The pH of a 0.20 M solution of KOH at 25°C isarrow_forwardPls help.arrow_forward
- Pls help.arrow_forward16) A 2.0 L flask containing 2.0 x 10-3 mol H2(g), 3.0 x 10-3 mol Cl2(g), and 4.0 x 10-3 mol HCl(g) at equilibrium. This system is represented by the following chemical equation: H2 (g) + Cl2 (g) → 2HCl(g) Calculate the equilibrium constant for this reaction.arrow_forward7) The pH of a 0.05M solution of HCl(aq) at 25°C is a. 1.3 b. 2.3 c. 3.3 d. 12.7arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co




