
Concept explainers
Suggest appropriate methods for preparing each of the following
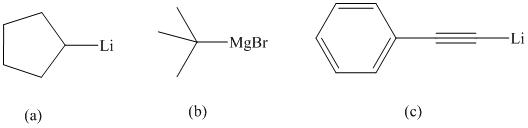
Interpretation:
The appropriate method of preparation for each of the given organometallic compounds is to be suggested.
Concept introduction:
The reaction of a metal with an organic halide is an oxidation–reduction in which the metal is the reducing agent.
lithium metal and magnesium metal in the presence of dry ether as a solvent, reacts with alkyl halides to produce the corresponding organo lithium and magnesium compounds respectively.
Organolithium reagents are used to prepare organometallic compounds analogous of terminal alkynes.
Organolithium compounds are strongly basic and react with terminal alkynes by abstracting the acidic proton and forming an alkane.
Answer to Problem 20P
Solution:

Explanation of Solution
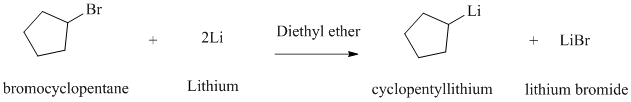
Alkyl halides react with lithium metal in the presence of dry ether as a solvent to produce the corresponding organo lithium compound.
The product given is cyclopentyl lithium. Reaction of cyclopentyl bromide with lithium metal in the presence of diethyl ether as a solvent will produce this cyclopentyl lithium.
The reaction is shown below:

The product given is tert-butyl magnesium bromide. Tert-butyl bromide react with magnesium metal in the presence of diethyl ether as a solvent will produce this tert-butyl magnesium bromide.
The reaction is shown below:

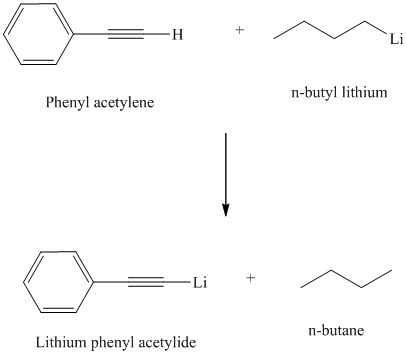
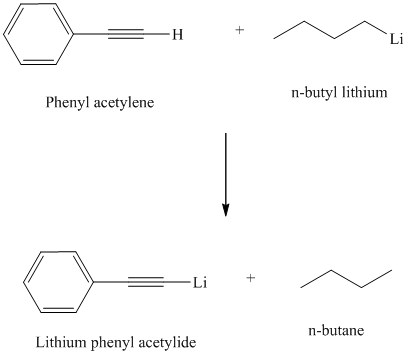
Organolithium reagents are used to prepare organometallic compounds analogous of terminal alkynes.
Organolithium compounds are strongly basic and react with terminal alkynes by abstracting the acidic proton and forming an alkane.
Thus, phenyl acetylene reacts with an organo lithium reagent like butyl lithium to produce lithium phenylacetylide and butane.
Want to see more full solutions like this?
Chapter 15 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- can someone draw out the reaction mechanism for this reaction showing all bonds, intermediates and side products Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided belowarrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure.arrow_forwardIdentify the missing organic reactants in the following reaction: X + Y H+ two steps Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Х :arrow_forward
- I am struggling with the IUPAC (sys H Reply ☑Mark as Unreadarrow_forwardDon't used hand raiting and don't used Ai solution and correct answerarrow_forwardH R Part: 1/2 :CI: is a/an electrophile Part 2 of 2 Draw the skeletal structure of the product(s) for the Lewis acid-base reaction. Include lone pairs and formal charges (if applicable) on the structures. 4-7: H ö- H Skip Part Check X :C1: $ % L Fi Click and drag to start drawing a structure. MacBook Pro & ㅁ x G 0: P Add or increase positive formal cha Save For Later Submit ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forward
- Draw the friedel-crafts acylation mechanism of m-Xylenearrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward1. Base on this experimental results, how do you know that the product which you are turning in is methyl 3-nitrobenzoate(meta substituted product ) rather than either of the other two products? 2. What observation suggests that at least a small amount of one or both of the other two isomers are in the mother liquor?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

