
Cyclobutadiene and (Cyclobutadiene)tricarbonyliron
As we saw in Section
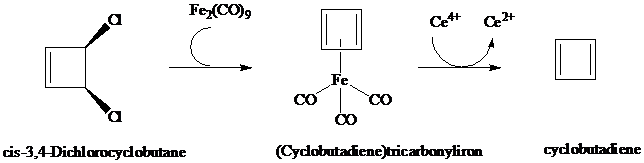
The tricarbonyliron complex of cyclobutadiene is sufficiently stable to undergo reactions
typical of


Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- So I need help with understanding how to solve these types of problems. I'm very confused on how to do them and what it is exactly, bonds and so forth that I'm drawing. Can you please help me with this and thank you very much!arrow_forwardSo I need help with this problem, can you help me please and thank you!arrow_forwardDraw the reaction mechanism to predict the product of the transformation below: N H ? H₂Oarrow_forward
- Draw a structural formula for the major product of the acid-base reaction shown. H 0 N + HCI (1 mole) CH3 N' (1 mole) CH3 You do not have to consider stereochemistry. ● • Do not include counter-ions, e.g., Na+, I, in your answer. . In those cases in which there are two reactants, draw only the product from 989 CH3 344 ? [Farrow_forwardQuestion 15 What is the major neutral organic product for the following sequence? 1. POCI₂ pyridine ? 2. OsO4 OH 3. NaHSO Major Organic Product ✓ OH OH 'OH OH 'OH 'CIarrow_forwardURGENT! PLEASE HELP!arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


