
Concept explainers
To review:
The classification of arginine as an essential amino acid in young animals.
Introduction:
Amino acids are amino-carboxylgroups containing
Explanation of Solution
Arginine is known to benon-essential amino acids in adult animals; this is because it is formed through the urea cycle present in the body. This cycle is not fully functional in young animals. This suggests that the formation of arginine with the help of this cycle within the body is hindered. This makes the amino acid an essential amino acid in young animals. In adults, the formation takes place in the following manner:
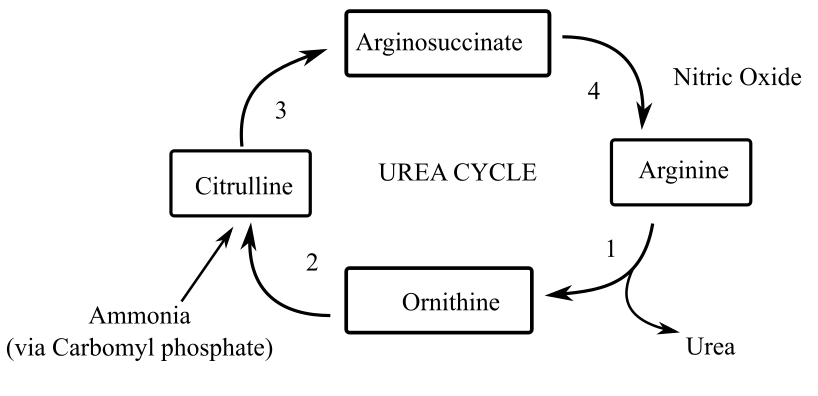
In the absence of urea cycle, it become difficult for young animals to synthesize arginine. This results in making it an essential dietary amino acid. Hence, this amino acid acts as an essential amino acid only in young animals. As soon as the animalgrows and the urea cycle begins, dietary intake of the amino acid is no longer required.
Therefore, it can be concluded that the lack of functionality of the urea cycle is the cause for making arginine an essential amino acid in infants. As soon as the cycle begins, with the growth of the organism, the amino acid is no longer an essential amino acid.
Want to see more full solutions like this?
Chapter 15 Solutions
Biochemistry, The Molecular Basis of Life, 6th Edition
- Do sensory neurons express ACE2 or only neurolipin-1 receptors for COVID19 virus particle binding?arrow_forwardExplain the process of CNS infiltration of COVID19 through sensory neurons from beginning to end, including processes like endocytosis, the different receptors/proteins that are involved, how they are transported and released, etc.,arrow_forwardH2C CH2 HC-COOO CH2 ܘHO-C-13c-O isocitrate C-S-COA H213c CH2 C-OO 13C-S-COA CH2 C-00 the label will not be present in succinyl CoA C-S-COA succinyl-CoAarrow_forward
- A culture of kidneys cells contains all intermediates of the citric acid cycle. It is treated with an irreversible inhibitor of malate dehydrogenase, and then infused withglucose. Fill in the following list to account for the number of energy molecules that are formed from that one molecule of glucose in this situation. (NTP = nucleotidetriphosphate, e.g., ATP or GTP)Net number of NTP:Net number of NADH:Net number of FADH2:arrow_forward16. Which one of the compounds below is the final product of the reaction sequence shown here? OH A B NaOH Zn/Hg aldol condensation heat aq. HCI acetone C 0 D Earrow_forward2. Which one of the following alkenes undergoes the least exothermic hydrogenation upon treatment with H₂/Pd? A B C D Earrow_forward
- 6. What is the IUPAC name of the following compound? A) (Z)-3,5,6-trimethyl-3,5-heptadiene B) (E)-2,3,5-trimethyl-1,4-heptadiene C) (E)-5-ethyl-2,3-dimethyl-1,5-hexadiene D) (Z)-5-ethyl-2,3-dimethyl-1,5-hexadiene E) (Z)-2,3,5-trimethyl-1,4-heptadienearrow_forwardConsider the reaction shown. CH2OH Ex. CH2 -OH CH2- Dihydroxyacetone phosphate glyceraldehyde 3-phosphate The standard free-energy change (AG) for this reaction is 7.53 kJ mol-¹. Calculate the free-energy change (AG) for this reaction at 298 K when [dihydroxyacetone phosphate] = 0.100 M and [glyceraldehyde 3-phosphate] = 0.00300 M. AG= kJ mol-1arrow_forwardIf the pH of gastric juice is 1.6, what is the amount of energy (AG) required for the transport of hydrogen ions from a cell (internal pH of 7.4) into the stomach lumen? Assume that the membrane potential across this membrane is -70.0 mV and the temperature is 37 °C. AG= kJ mol-1arrow_forward
- Consider the fatty acid structure shown. Which of the designations are accurate for this fatty acid? 17:2 (48.11) 18:2(A9.12) cis, cis-A8, A¹¹-octadecadienoate w-6 fatty acid 18:2(A6,9)arrow_forwardClassify the monosaccharides. H-C-OH H. H-C-OH H-C-OH CH₂OH H-C-OH H-C-OH H-C-OH CH₂OH CH₂OH CH₂OH CH₂OH D-erythrose D-ribose D-glyceraldehyde Dihydroxyacetone CH₂OH CH₂OH C=O Answer Bank CH₂OH C=0 HO C-H C=O H-C-OH H-C-OH pentose hexose tetrose H-C-OH H-C-OH H-C-OH aldose triose ketose CH₂OH CH₂OH CH₂OH D-erythrulose D-ribulose D-fructosearrow_forwardFatty acids are carboxylic acids with long hydrophobic tails. Draw the line-bond structure of cis-A9-hexadecenoate. Clearly show the cis-trans stereochemistry.arrow_forward
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning- Essentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning





