
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
11th Edition
ISBN: 9781305705159
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.26P
15-26 For centuries, Chinese herbal medicine has
used extracts oi Ephedra sinica to treat asthma. The asthma-relieving component of this plant is ephedrine, a very potent dilator of the air passages of the lungs. The naturally occurring stereoisomer is levorotatory and has the following structure.
HO ¥ Nhch3
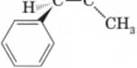
Ephedrine |n|„ = —41°
- Mark each stereocenter in epinephrine with an asterisk.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Illustrate reaction mechanisms of
alkenes with water in the presence of
H2SO4, detailing each step of the
process. Please show steps of
processing. Please do both, I will
thumb up for sure
#1
#3
Draw the following molecule: (Z)-1-chloro-1-butene
Identify the molecule as having a(n) E, Z, cis, or trans configuration.
CH3
H₁₂C
○ E
○ z
○ cis
trans
Chapter 15 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
Ch. 15.1 - Prob. 15.1PCh. 15.2 - Problem 15-2 Assign priorities to the groups in...Ch. 15.2 - Problem 15-3 Assign an R or S configuration to the...Ch. 15.3 - Problem 15-4 3-Amino-2-butanol has two...Ch. 15.3 - Prob. 15.5PCh. 15.3 - Prob. 15.6PCh. 15 - 15-7 Answer true or false. The cis and trans...Ch. 15 - 15-8 What does the term “chiral” mean? Give an...Ch. 15 - 15-9 What does the term “achiral” mean? Give an...Ch. 15 - 15-10 Define the term “stereoisomer.” Name three...
Ch. 15 - 15-11 In what way are constitutional isomers...Ch. 15 - 15-12 Which of the following objects are chiral...Ch. 15 - Prob. 15.13PCh. 15 - Prob. 15.14PCh. 15 - 15-15 Explain why the carbon of a carbonyl group...Ch. 15 - 15-16 Which of the following compounds contain...Ch. 15 - 15-17 Which of the following compounds contain...Ch. 15 - Prob. 15.18PCh. 15 - 15-19 Draw the mirror image for each molecule: OH...Ch. 15 - Prob. 15.20PCh. 15 - 15-21 Answer true or false. For a molecule with...Ch. 15 - Prob. 15.22PCh. 15 - Prob. 15.23PCh. 15 - Prob. 15.24PCh. 15 - Prob. 15.25PCh. 15 - 15-26 For centuries, Chinese herbal medicine has...Ch. 15 - Prob. 15.27PCh. 15 - Prob. 15.28PCh. 15 - Prob. 15.29PCh. 15 - 15-30 (Chemical Connections 15A) What does it mean...Ch. 15 - Prob. 15.31PCh. 15 - Prob. 15.32PCh. 15 - Prob. 15.33PCh. 15 - Prob. 15.34PCh. 15 - 15-35 Following are structural formulas for three...Ch. 15 - Prob. 15.36PCh. 15 - 15-37 Consider a cyclohexane ring substituted with...Ch. 15 - Prob. 15.38PCh. 15 - Prob. 15.39PCh. 15 - Prob. 15.40PCh. 15 - 15-41 Compound A(C5Hh, is not optically active and...Ch. 15 - Prob. 15.42PCh. 15 - 15-43 Triamcinolone acetonide, the active...Ch. 15 - 15-44 Consider the structure of the...Ch. 15 - Prob. 15.45PCh. 15 - 15-46 Consider Lunesta, a nonbenzodiazepine...Ch. 15 - Prob. 15.47P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the molecule as having a(n) E, Z, cis, or trans configuration. H₂C- CH3 О Е ○ cis ○ transarrow_forwardThe decomposition of dinitrogen pentoxide according to the equation: 50°C 2 N2O5(g) 4 NO2(g) + O2(g) follows first-order kinetics with a rate constant of 0.0065 s-1. If the initial concentration of N2O5 is 0.275 M, determine: the final concentration of N2O5 after 180 seconds. ...arrow_forwardDon't used hand raitingarrow_forward
- CS2(g) →CS(g) + S(g) The rate law is Rate = k[CS2] where k = 1.6 × 10−6 s−¹. S What is the concentration of CS2 after 5 hours if the initial concentration is 0.25 M?arrow_forwardCS2(g) → CS(g) + S(g) The rate law is Rate = k [CS2] where k = 1.6 × 10-6 s−1. S Calculate the half-life.arrow_forwardThe following is a first order reaction where the rate constant, k, is 6.29 x 10-3 min-*** What is the half-life? C2H4 C2H2 + H2arrow_forward
- Control Chart Drawing Assignment The table below provides the number of alignment errors observed during the final inspection of a certain model of airplane. Calculate the central, upper, and lower control limits for the c-chart and draw the chart precisely on the graph sheet provided (based on 3-sigma limits). Your chart should include a line for each of the control limits (UCL, CL, and LCL) and the points for each observation. Number the x-axis 1 through 25 and evenly space the numbering for the y-axis. Connect the points by drawing a line as well. Label each line drawn. Airplane Number Number of alignment errors 201 7 202 6 203 6 204 7 205 4 206 7 207 8 208 12 209 9 210 9 211 8 212 5 213 5 214 9 215 8 216 15 217 6 218 4 219 13 220 7 221 8 222 15 223 6 224 6 225 10arrow_forwardCollagen is used to date artifacts. It has a rate constant = 1.20 x 10-4 /years. What is the half life of collagen?arrow_forwardיווי 10 20 30 40 50 60 70 3.5 3 2.5 2 1.5 1 [ppm] 3.5 3 2.5 2 1.5 1 6 [ppm] 1 1.5 -2.5 3.5arrow_forward
- 2H2S(g)+3O2(g)→2SO2(g)+2H2O(g) A 1.2mol sample of H2S(g) is combined with excess O2(g), and the reaction goes to completion. Question Which of the following predicts the theoretical yield of SO2(g) from the reaction? Responses 1.2 g Answer A: 1.2 grams A 41 g Answer B: 41 grams B 77 g Answer C: 77 grams C 154 g Answer D: 154 grams Darrow_forwardPart VII. Below are the 'HNMR, 13 C-NMR, COSY 2D- NMR, and HSQC 2D-NMR (similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H1003 - Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 f1 (ppm) Predicted 13C NMR Spectrum 100 f1 (ppm) 30 220 210 200 190 180 170 160 150 140 130 120 110 90 80 70 -26 60 50 40 46 30 20 115 10 1.0 0.9 0.8 0 -10arrow_forwardQ: Arrange BCC and Fec metals, in sequence from the Fable (Dr. R's slides) and Calculate Volume and Density. Aa BCC V 52 5 SFCCarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY