
Concept explainers
15-44 Consider the structure of the immunosuppressant FK-506, a molecule shown to disrupt calcineurin- mediated signal transduction in T-lymphocytes.
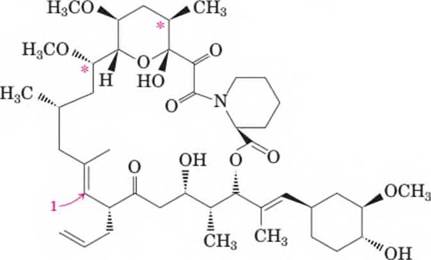
- What is the molecular formula of this immunosuppressant?
- How manj' stereocenters are present in FK-506? Determine the maximum number of stereoisomers possible.
(a)
Interpretation:
The molecular formula of FK-506 molecule should be determined.
Concept Introduction:
A molecular formula represents the exact number of atoms present for each element in the compound.
Answer to Problem 15.44P
The molecular formula of FK-506 is C44 H69 NO12..
Explanation of Solution
The total number of C, H, n and O in the given structure is:
The total number of carbon = 44.
The total number of hydrogen = 69.
The total number of oxygen = 12.
The total number of nitrogen = 1.
Hence, the molecular formula is C44 H69 NO12..
(b)
Interpretation:
The number of stereocenters and the maximum number of stereoisomers possible for FK-506 molecule should be determined.
Concept Introduction:
FK-506 molecule contains various functional groups and also has stereocenters which are the carbon atoms bonded with four different atoms or group of atom. The number of stereoisomers can be calculated with the help of 2n formula. Here ‘n’ indicates the number of stereocenters in the molecule.
Answer to Problem 15.44P
The stereocenters are shown by “*”:
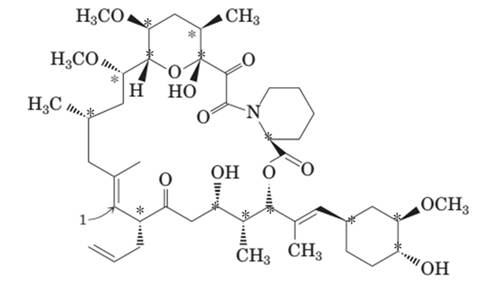
There are 14 stereocenters hence 214 = 16384 stereoisomers will be there.
Explanation of Solution
A stereocenter atom is that atom in a molecule which is connected to four different group of atoms or atom(s).
There are 14 stereocenters in FK-506 molecule as shown by “*”:
The formula for total stereoisomers is 2n hence there will be total 214 = 16384 stereoisomers in the molecule.
(c)
Interpretation:
Various functional groups in FK-506 molecule should be identified and labeled.
Concept Introduction:
An atom or group of atoms due to which a compound shows the characteristic properties is said to be the functional group.
Answer to Problem 15.44P
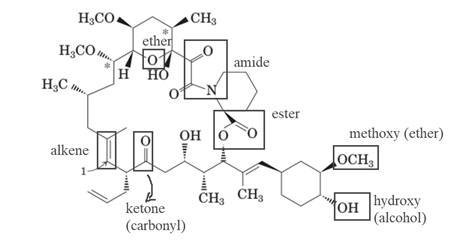
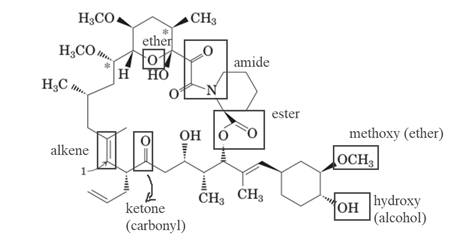
The molecule of FK-506 contains:
- Alkene group
- Carbonyl group (ketone)
- Alcohol group (hydroxyl)
- Ester group
- Methoxy group (ether)
- Amide group
Explanation of Solution
The molecule of FK-506 contains alkene group, carbonyl group (ketone), alcohol group (hydroxyl), ester group, methoxy group (ether) and amide group.
(d)
Interpretation:
The absolute configuration of the two stereocenters labeled with asterisks (*) in FK-506 molecule should be determined.
Concept Introduction:
FK-506 molecule contains various functional groups and also have stereocenters which are the carbon atoms bonded with four different atoms or group of atom. The number of stereoisomers can be calculate with the help of 2n formula. Here ‘n’ indicates the number of stereocenters in the molecule.
Answer to Problem 15.44P
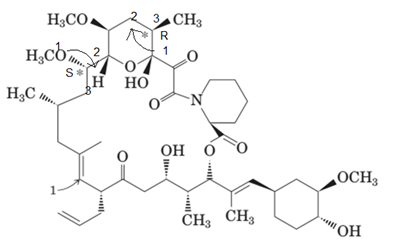
The marked stereocenters are r and s respectively.
Explanation of Solution
To assign the absolute configuration to C* we need to check the bonded group to this carbon atom. Then give numbers according to their atomic masses. If the movement from number 1 to 3 is clockwise; it will be r and if it will be anticlockwise, it will be s configuration.
(e)
Interpretation:
Whether FK-506 molecule is soluble in ethanol or not should be determined.
Concept Introduction:
FK-506 molecule contains various functional groups and also have stereocenters which are the carbon atoms bonded with four different atoms or group of atom. The number of stereoisomers can be calculate with the help of 2n formula. Here ‘n’ indicates the number of stereocenters in the molecule.
Answer to Problem 15.44P
FK-506 is insoluble in water whereas moderatelysoluble in ethanol and other organic solvents because it is bulky structure.
Explanation of Solution
The organic compounds which cannot form hydrogen-bond with water are insoluble in water. Usually organic compounds are soluble in organic solvents like ethanol. Here FK-506 has bulky structure therefore it is unable to form hydrogen bonds with water and water insoluble but soluble in organic solvents like ethanol.
(f)
Interpretation:
The geometry of carbon atom labeled “1” in FK-506 molecule should be determined.
Concept Introduction:
FK-506 molecule contains various functional groups and also have stereocenters which are the carbon atoms bonded with four different atoms or group of atom. The number of stereoisomers can be calculate with the help of 2n formula. Here ‘n’ indicates the number of stereocenters in the molecule.
Answer to Problem 15.44P
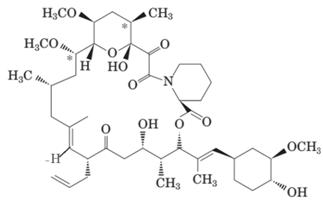
Here H and CH3 are in opposite directions therefore it has E-configuration. Here the C-1 carbon atom has double bond therefore it must have sp2 hybridization.
Explanation of Solution
The double bonded carbon atom is always sp2 hybridized hence here C-1 will be sp2 hybridized. If the groups of same priority are on same side; it will be Z-configuration whereas the groups of same priority on opposite side will be E-configuration. Here H and CH3 have low priority are on opposite sides hence it is E-configuration.
(g)
Interpretation:
The alternative chair conformations of the cyclohexane ring at the lower right of FK-506 should be drawn and more stable conformation should be labeled.
Concept Introduction:
FK-506 molecule contains various functional groups and also have stereocenters which are the carbon atoms bonded with four different atoms or group of atom. The number of stereoisomers can be calculate with the help of 2n formula. Here ‘n’ indicates the number of stereocenters in the molecule.
Answer to Problem 15.44P
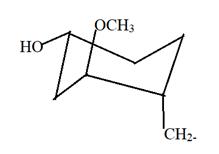
Here this will be most stable conformation as least repulsion between all bonded groups is there.
Explanation of Solution
Here in the given structure of FK-506; there is one cyclohexane ring with OCH3, OH and other C chain at 1, 3 and 4 position. Hence this will be most stable conformation as least repulsion between all bonded groups is there.
(h)
Interpretation:
Whether there is any aromatic component present in FK-506 molecule or not should be determined.
Concept Introduction:
The ring structures of the compound having uncommon stability due to delocalized pi electron density shared in between all the carbon atoms of the ring is said to be an aromatic compound.
Answer to Problem 15.44P
There is no aromatic component in the molecule.
Explanation of Solution
There is no conjugated pi system or benzene ring is present; hence there is no aromatic component in the molecule.
(i)
Interpretation:
Whether the enantiomer of FK-506 has same side effects as that of FK-506 should be determined.
Concept Introduction:
FK-506 molecule contains various functional groups and also have stereocenters which are the carbon atoms bonded with four different atoms or group of atom. The number of stereoisomers can be calculate with the help of 2n formula. Here ‘n’ indicates the number of stereocenters in the molecule.
Answer to Problem 15.44P
Yes. enantiomers of FK-506 also report same side effects as enantiomers exhibit same physical and chemical properties; only differ in their optical properties.
Explanation of Solution
Enantiomers are optical isomers with same chemical and physical properties hence enantiomers of FK-506 also show same side effects.
Want to see more full solutions like this?
Chapter 15 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- Curved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the structure of the missing reactants, intermediates, or products in the following mechanism. Include all lone pairs. Ignore stereochemistry. Ignore inorganic byproducts. H Br2 (1 equiv) H- Select to Draw Starting Alkene Draw Major Product I I H2O 四: ⑦.. Q Draw Major Charged Intermediate Iarrow_forwardNH (aq)+CNO (aq) → CO(NH2)2(s) Experiment [NH4] (M) [CNO] (M) Initial rate (M/s) 1 0.014 0.02 0.002 23 0.028 0.02 0.008 0.014 0.01 0.001 Calculate the rate contant for this reaction using the data provided in the table.arrow_forward2CIO2 + 20H-1 CIO31 + CIO2 + H2O Experiment [CIO2], M [OH-1], M 1 0.0500 0.100 23 2 0.100 0.100 3 0.100 0.0500 Initial Rate, M/s 0.0575 0.230 0.115 ... Given this date, calculate the overall order of this reaction.arrow_forward
- 2 3 .(be)_[Ɔ+(be)_OI ← (b²)_IƆO+ (be)_I Experiment [1-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 0.000069 4 0.0025 0.0025 0.000140 Calculate the rate constant of this reaction using the table data.arrow_forward1 2 3 4 I(aq) +OCl(aq) → IO¯¯(aq) + Cl¯(aq) Experiment [I-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 Calculate the overall order of this reaction using the table data. 0.0025 0.000069 0.0025 0.000140arrow_forwardH2O2(aq) +3 I¯(aq) +2 H+(aq) → 13(aq) +2 H₂O(l)· ••• Experiment [H2 O2]o (M) [I]o (M) [H+]。 (M) Initial rate (M/s) 1 0.15 0.15 0.05 0.00012 234 0.15 0.3 0.05 0.00024 0.3 0.15 0.05 0.00024 0.15 0.15 0.1 0.00048 Calculate the overall order of this reaction using the table data.arrow_forward
- The U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m³ Part A If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.40 L.) ΜΕ ΑΣΦ = 2.35 1013 ? atoms ! Check your rounding. Your final answer should be rounded to 2 significant figures in the last step. No credit lost. Try again.arrow_forwardY= - 0.039 (14.01) + 0.7949arrow_forwardSuppose 1.76 g of magnesium acetate (Mg (CH3CO2)2) are dissolved in 140. mL of water. Find the composition of the resulting electrolyte solution. In particular, list the chemical symbols (including any charge) of each dissolved ion in the table below. List only one ion per row. mEq Then, calculate the concentration of each ion in dwrite the concentration in the second column of each row. Be sure you round your answers to the L correct number of significant digits. ion Add Row mEq L x 5arrow_forward
- A pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows.arrow_forwardNonearrow_forwardNonearrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
