
(a)
Interpretation:
All the stereocentres should be marked using asterisk in the given molecule.
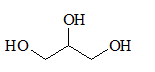
Concept Introduction:
The stereocentre is generated in an organic compound due to presence of chiral carbon.
The chiral carbon is the carbon bearing all the four groups different. Even isotopes are considered as different groups.
(b)
Interpretation:
All the stereocentres should be marked using asterisk in the given molecule.
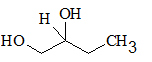
Concept Introduction:
The stereocentre is generated in an organic compound due to presence of chiral carbon.
The chiral carbon is the carbon bearing all the four groups different. Even isotopes are considered as different groups.
(c)
Interpretation:
All the stereocentres should be marked using asterisk in the given molecule.
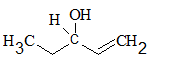
Concept Introduction:
The stereocentre is generated in an organic compound due to presence of chiral carbon.
The chiral carbon is the carbon bearing all the four groups different. Even isotopes are considered as different groups.
(d)
Interpretation:
All the stereocentres should be marked using asterisk in the given molecule.
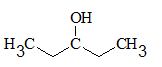
Concept Introduction:
The stereocentre is generated in an organic compound due to presence of chiral carbon.
The chiral carbon is the carbon bearing all the four groups different. Even isotopes are considered as different groups.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- Can I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





