
(a)
Interpretation:
The
Concept introduction:
Dienes are the compounds that contain carbon-carbon double bonds. The term conjugated dienes is used when the double bonds are present alternatively in a hydrocarbon chain. The mathematical function that describes the wave like behavior of the electron in a molecule is expressed by molecular orbital. The physical and chemical properties are found with the help of molecular orbital.
Answer to Problem 15.1P
The bonding molecular orbitals are
The anti-bonding molecular orbitals are
The molecular orbitals of conjugated
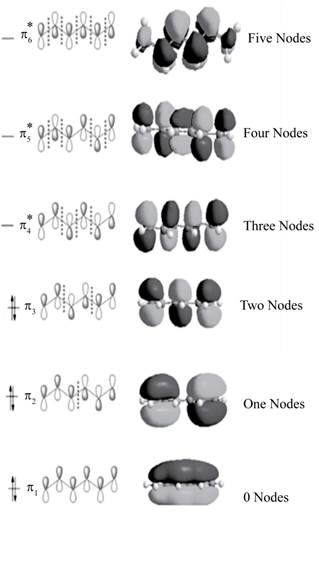
Explanation of Solution
In
The three bonding molecular orbitals are
The three anti bonding molecular orbitals are
The sketch showing the molecular orbital of conjugated
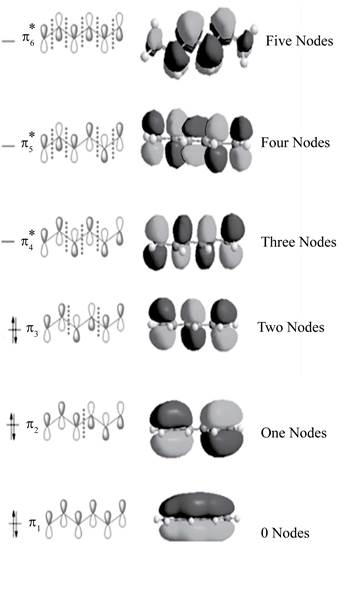
Figure 1
The three bonding molecular orbitals are
The three anti bonding molecular orbitals are
The sketch showing the molecular orbital of conjugated
(b)
Interpretation:
The total number of nodes in the
Concept introduction:
The term conjugated dienes is used when the double bonds are present alternatively in a hydrocarbon chain. The mathematical function that describes the wave like behavior of an electron in a molecule is expressed by molecular orbital. The molecular orbital contains nodes and lobes. Nodes are the regions in the molecular orbital of conjugated system in which the adjoining orbital lobes undergoes a phase change.
Answer to Problem 15.1P
The total number of nodes that the molecular orbital (MOs) of conjugated
Explanation of Solution
There is no change of phase occurs in lobes in the
There is one change of phase occurs in lobes in the
There is two change of phase occurs in lobes in the
There are four change of phase occurs in lobes in the
There are five change of phase occurs in lobes in the
There are six change of phase occurs in lobes in the
The number of nodes that molecular orbital (MOs) of conjugated
| Molecular Orbital | Number of Nodes |
| Zero | |
| One | |
| Two | |
| Three | |
| Four | |
| Five |
The number of nodes that molecular orbital (MOs) of conjugated
(c)
Interpretation:
The position of the nodes in
Concept introduction:
The term conjugated dienes is used when the double bonds are present alternatively in a hydrocarbon chain. The mathematical function that describes the wave like behavior of the electron in a molecule is expressed by molecular orbital. The molecular orbital contains nodes and lobes. Nodes are the regions in the molecular orbital of conjugated system in which the adjoining orbital lobes undergoes a phase change.
Answer to Problem 15.1P
The position of the nodes in
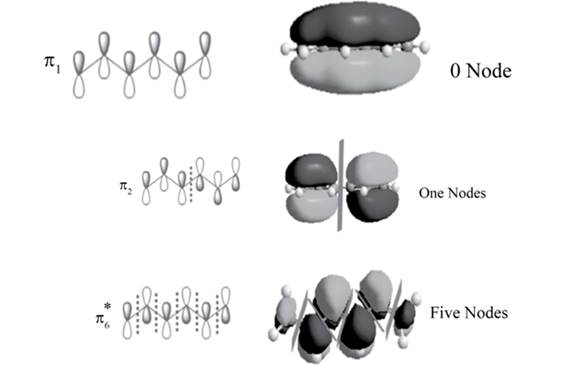
Explanation of Solution
There is no change of phase occurs in lobes in the
.
The sketch of
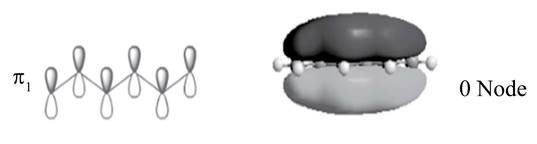
Figure 2
There is one change of phase occurs in lobes in the
The sketch of

Figure 3
There are 6 change of phase occurs in lobes in the
The
The sketch of

Figure 4
The position of the nodes in
Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry
- 1. For each of the reaction "railroads" below, you are either asked to give the structure(s) of the starting material(s) or product(s), or provide reagents/conditions to accomplish the transformation, as indicated by the boxes. a. NaOMe H+ .CO,H HO₂C MeOH (excess) MeOH H3C Br يع CH3 1. LiAlH4 2. H3O+ 3. PBг3 H3C 1. Et-Li 2. H3O+ -CO₂Me -CO₂Me OH CH3 CH3 ল CH3arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe ག1, ད།་, - + H You can draw 1 and 2 in any arrangement you like. 2 work up Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Parrow_forwardWhat is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forward
- ΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forwardHow many hydrogen atoms are connected to the indicated carbon atom?arrow_forwardIdentify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forward
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
