
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.15QAP
The following lifetimes were measured for the chloride quenching of quinine sulfate given in Example 15-1.The fluorescence intensities are given in the example.
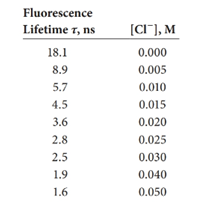
(a) Plot fluorescence intensity versus [Cl-].
(b) Plot the ratio of intensity to lifetime, F-
(c) Develop a normalization factor to correct the measured fluorescence intensity to that of the solution without quencher.
(d) Plot on the same graph Fversus [Cl-] and Fcorr versus (CI-].
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Basic strength of organic bases.
Nucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See image
Predict the final product. If 2 products are made, list which should be “major” and “minor” *see attached
Chapter 15 Solutions
Principles of Instrumental Analysis
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Photochemistry : Introduction to Basic Theory of Photochemical Process [Part 1]; Author: Dr. Vikrant Palekar;https://www.youtube.com/watch?v=2NDOL11d6no;License: Standard YouTube License, CC-BY
Photochemistry-1; Author: CH-08:ARYABHATT [Mathematics, Physics, Chemistry];https://www.youtube.com/watch?v=DC4J0t1z3e8;License: Standard Youtube License