
Chemistry
9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 113CP
When a diprotic acid. H2A. is titrated with NaOH, the protons on the diprotic acid are generally removed one at a time, resulting in a pH curve that has the following generic shape:
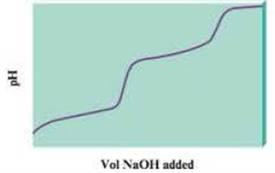
- a. Notice that the plot has essentially two titration curves. If the first equivalence point occurs at 100.0 mL NaOH added, what volume of NaOH added corresponds to the second equivalence point?
- b. For the following volumes of NaOH added, list the major species present after the OH– reacts completely.
- i. 0 mL NaOH added
- ii. between 0 and 100.0 mL NaOH added
- iii. 100.0 mL NaOH added
- iv. between 100.0 and 200.0 mL NaOH added
- v. 200.0 mL NaOH added
- vi. after 200.0 mL NaOH added
- c. If die pH at 50.0 mL NaOH added is 4.0 and the pH at 150.0 mL NaOH added is 8.0, determine the values Ka and Ka. for the diprotic acid.
- d.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Basic strength of organic bases.
Nucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See image
Predict the final product. If 2 products are made, list which should be “major” and “minor” *see attached
Chapter 15 Solutions
Chemistry
Ch. 15 - What is meant by the presence of a common ion? How...Ch. 15 - Define a buffer solution. What makes up a buffer...Ch. 15 - One of the most challenging parts of solving...Ch. 15 - A good buffer generally contains relatively equal...Ch. 15 - Draw the general titration curve for a strong acid...Ch. 15 - Instead of the titration of a strong acid by a...Ch. 15 - Sketch the titration curve for a weak acid...Ch. 15 - Sketch the titration curve for a weak base...Ch. 15 - What is an acidbase indicator? Define the...Ch. 15 - Why does an indicator change from its acid color...
Ch. 15 - What are the major species in solution after...Ch. 15 - A friend asks the following: Consider a buffered...Ch. 15 - Mixing together solutions of acetic acid and...Ch. 15 - Could a buffered solution be made by mixing...Ch. 15 - Sketch two pH curves, one for the titration of a...Ch. 15 - Sketch a pH curve for the titration of a weak acid...Ch. 15 - You have a solution of the weak acid HA and add...Ch. 15 - You have a solution of the weak acid HA and add...Ch. 15 - The common ion effect for weak acids is to...Ch. 15 - Prob. 10QCh. 15 - A best buffer has about equal quantities of weak...Ch. 15 - Consider the following pH curves for 100.0 mL of...Ch. 15 - An acid is titrated with NaOH. The following...Ch. 15 - Consider the following four titrations. i. 100.0...Ch. 15 - Figure 14-4 shows the pH curves for the titrations...Ch. 15 - Acidbase indicators mark the end point of...Ch. 15 - How many of the following are buffered solutions?...Ch. 15 - Which of the following can be classified as buffer...Ch. 15 - A certain buffer is made by dissolving NaHCO3 and...Ch. 15 - A buffer is prepared by dissolving HONH2 and...Ch. 15 - Calculate the pH of each of the following...Ch. 15 - Calculate the pH of each of the following...Ch. 15 - Compare the percent dissociation of the acid in...Ch. 15 - Compare the percent ionization of the base in...Ch. 15 - Calculate the pH after 0.020 mole of HCl is added...Ch. 15 - Calculate the pH after 0.020 mole of HCl is added...Ch. 15 - Calculate the pH after 0.020 mole of NaOH is added...Ch. 15 - Calculate the pH after 0.020 mole of NaOH is added...Ch. 15 - Which of the solutions in Exercise 21 shows the...Ch. 15 - Prob. 30ECh. 15 - Calculate the pH of a solution that is 1.00 M HNO2...Ch. 15 - Calculate the pH of a solution that is 0.60 M HF...Ch. 15 - Calculate the pH after 0.10 mole of NaOH is added...Ch. 15 - Calculate the pH after 0.10 mole of NaOH is added...Ch. 15 - Calculate the pH of each of the following buffered...Ch. 15 - Calculate the pH of each of the following buffered...Ch. 15 - Calculate the pH of a buffered solution prepared...Ch. 15 - A buffered solution is made by adding 50.0 g NH4Cl...Ch. 15 - Calculate the pH after 0.010 mole of gaseous HCl...Ch. 15 - An aqueous solution contains dissolved C6H5NH3Cl...Ch. 15 - Calculate the mass of sodium acetate that must be...Ch. 15 - What volumes of 0.50 M HNO2 and 0.50 M NaNO2 must...Ch. 15 - Consider a solution that contains both C5H5N and...Ch. 15 - Calculate the ratio [NH3]/[NH4+] in...Ch. 15 - Carbonate buffers are important in regulating the...Ch. 15 - When a person exercises, muscle contractions...Ch. 15 - Consider the acids in Table 13-2. Which acid would...Ch. 15 - Consider the bases in Table 13-3. Which base would...Ch. 15 - Calculate the pH of a solution that is 0.40 M...Ch. 15 - Calculate the pH of a solution that is 0.20 M HOCl...Ch. 15 - Which of the following mixtures would result in...Ch. 15 - Which of the following mixtures would result in a...Ch. 15 - What quantity (moles) of NaOH must be added to 1.0...Ch. 15 - Calculate the number of moles of HCl(g) that must...Ch. 15 - Consider the titration of a generic weak acid HA...Ch. 15 - Sketch the titration curve for the titration of a...Ch. 15 - Consider the titration of 40.0 mL of 0.200 M HClO4...Ch. 15 - Consider the titration of 80.0 mL of 0.100 M...Ch. 15 - Consider the titration of 100.0 mL of 0.200 M...Ch. 15 - Consider the titration of 100.0 mL of 0.100 M...Ch. 15 - Lactic acid is a common by-product of cellular...Ch. 15 - Repeat the procedure in Exercise 61, but for the...Ch. 15 - Repeat the procedure in Exercise 61, but for the...Ch. 15 - Repeat the procedure in Exercise 61, but for the...Ch. 15 - Calculate the pH at the halfway point and at the...Ch. 15 - In the titration of 50.0 mL of 1.0 M methylamine,...Ch. 15 - You have 75.0 mL of 0.10 M HA. After adding 30.0...Ch. 15 - A student dissolves 0.0100 mole of an unknown weak...Ch. 15 - Two drops of indicator HIn (Ka = 1.0 109), where...Ch. 15 - Methyl red has the following structure: It...Ch. 15 - Potassium hydrogen phthalate, known as KHP (molar...Ch. 15 - A certain indicator HIn has a pKa of 3.00 and a...Ch. 15 - Which of the indicators in Fig. 14-8 could be used...Ch. 15 - Prob. 74ECh. 15 - Which of the indicators in Fig. 14-8 could be used...Ch. 15 - Prob. 76ECh. 15 - Estimate the pH of a solution in which bromcresol...Ch. 15 - Estimate the pH of a solution in which crystal...Ch. 15 - A solution has a pH of 7.0. What would be the...Ch. 15 - A solution has a pH of 4.5. What would be the...Ch. 15 - Derive an equation analogous to the...Ch. 15 - a. Calculate the pH of a buffered solution that is...Ch. 15 - Tris(hydroxymethyl)aminomethane, commonly called...Ch. 15 - You make 1.00 L of a buffered solution (pH = 4.00)...Ch. 15 - You have the following reagents on hand: Solids...Ch. 15 - Prob. 86AECh. 15 - Phosphate buffers are important in regulating the...Ch. 15 - What quantity (moles) of HCl(g) must be added to...Ch. 15 - Prob. 89AECh. 15 - The following plot shows the pH curves for the...Ch. 15 - Calculate the volume of 1.50 102 M NaOH that must...Ch. 15 - Prob. 92AECh. 15 - A certain acetic acid solution has pH = 2.68....Ch. 15 - A 0.210-g sample of an acid (molar mass = 192...Ch. 15 - The active ingredient in aspirin is...Ch. 15 - One method for determining the purity of aspirin...Ch. 15 - A student intends to titrate a solution of a weak...Ch. 15 - A student titrates an unknown weak acid, HA, to a...Ch. 15 - A sample of a certain monoprotic weak acid was...Ch. 15 - Consider 1.0 L of a solution that is 0.85 M HOC6H5...Ch. 15 - What concentration of NH4Cl is necessary to buffer...Ch. 15 - Consider the following acids and bases: HCO2H Ka =...Ch. 15 - Consider a buffered solution containing CH3NH3Cl...Ch. 15 - Consider the titration of 150.0 mL of 0.100 M HI...Ch. 15 - Consider the titration of 100.0 mL of 0.100 M HCN...Ch. 15 - Consider the titration of 100.0 mL of 0.200 M...Ch. 15 - Consider the following four titrations (iiv): i....Ch. 15 - Another way to treat data from a pH titration is...Ch. 15 - A buffer is made using 45.0 mL of 0.750 M HC3H5O2...Ch. 15 - A 0.400-M solution of ammonia was titrated with...Ch. 15 - What volume of 0.0100 M NaOH must be added to 1.00...Ch. 15 - Consider a solution formed by mixing 50.0 mL of...Ch. 15 - When a diprotic acid. H2A. is titrated with NaOH,...Ch. 15 - Prob. 114CPCh. 15 - The titration of Na2CO3 with HCl bas the following...Ch. 15 - Consider the titration curve in Exercise 115 for...Ch. 15 - A few drops of each of the indicators shown in the...Ch. 15 - Malonic acid (HO2CCH2CO2H) is a diprotic acid. In...Ch. 15 - A buffer solution is prepared by mixing 75.0 mL of...Ch. 15 - A 10.00-g sample of the ionic compound NaA, where...Ch. 15 - Calculate the pH of a solution prepared by mixing...Ch. 15 - Consider a solution prepared by mixing the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY