
Concept explainers
(a)
Interpretation:
The statement “ethyl butanoate is an ether” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(a)
Answer to Problem 4MCP
The given statement is false because ethyl butanoate is an ester of ethanol and butanoic acid.
Explanation of Solution
Ethyl butanoate is an ester, which can be synthesized from butanoic acid and ethanol.
The
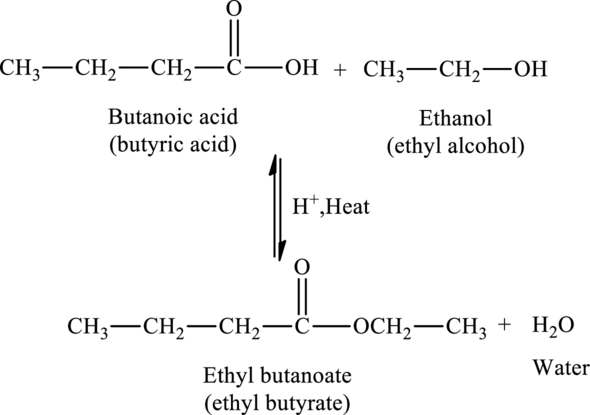
The statement “ethyl butanoate is an ether” is false because ethyl butanoate is an ester of ethanol and butanoic acid.
(b)
Interpretation:
The statement “ethyl butanoate has a higher melting point than hexanoic acid” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(b)
Answer to Problem 4MCP
The given statement is false because ethyl butanoate is lower melting point than hexanoic acid.
Explanation of Solution
Hexanoic acid is a
Carboxylic acids have strong intermolecular hydrogen bonding with each other and strong dipole-dipole attractions.
The presence of polar carboxyl group and intermolecular hydrogen bonding makes these acids have higher boiling points. The presence of dimers increases the strength of the van der Waals dispersion forces, which makes them have high boiling points.
Ester does not engage in hydrogen bonding with each other, but they form hydrogen bonds with water.
Hence, the statement ethyl butanoate has a higher melting point than hexanoic acid” is false because hexanoic acid has strong intermolecular hydrogen bonding and strong dipole-dipole attractions when compared to ethyl butanoate.
(c)
Interpretation:
The statement “ethyl butanoate forms hydrogen bonds with water” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(c)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
Esters can form hydrogen bonds with water. The hydrogen bonds are formed through their oxygen atoms to the hydrogen atoms of water. Thus, esters are slightly soluble in water.
(d)
Interpretation:
The statement “ethyl butanoate is nonpolar” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(d)
Answer to Problem 4MCP
The given statement is false because ethyl butanoate is slightly polar.
Explanation of Solution
The structure of ethyl butanoate is,
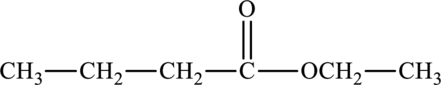
The carbonyl group present in ethyl butanoate is polar in nature and thereby can participate in dipole-dipole attractions. Therefore, ethyl butanoate is polar in nature.
The statement “ethyl butanoate is nonpolar” is false due to the presence of polar carbonyl group in them; ethyl butanoate is polar in nature.
(e)
Interpretation:
The statement “ethyl butanoate has a molecular formula of
(e)
Answer to Problem 4MCP
The given statement is false because ethyl butanoate has a molecular formula of
Explanation of Solution
The structure of ethyl butanoate is,
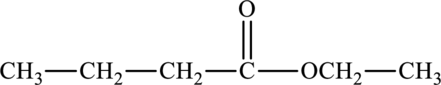
From the structure of ethyl butanoate, it can be seen that ethyl butanoate comprises of six carbon atoms, twelve hydrogen atoms and two oxygen atoms. Hence the molecular formula of ethyl butanoate is
The statement “ethyl butanoate has a molecular formula of
(f)
Interpretation:
The statement “ethyl butanoate has a higher boiling point than hexanol” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(f)
Answer to Problem 4MCP
The given statement is false because hexanol has higher boiling point than ethyl butanoate. The lack of strong intermolecular hydrogen bonding makes ethyl butanoate have less boiling point.
Explanation of Solution
Alcohols have strong intermolecular hydrogen bonding. The
Ester does not engage in hydrogen bonding with each other, but they form hydrogen bonds with water.
The absence of strong intermolecular hydrogen bonding makes ethyl butanoate have lesser boiling point than hexanol. Hence, the statement “ethyl butanoate has a higher boiling point than hexanol” is false.
(g)
Interpretation:
The statement “ethyl butanoate is a liquid at room temperature” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(g)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
Ethyl butanoate is clear colorless liquid with a pineapple-like odor. It is less dense than water and insoluble in water. It has a fruity odor, similar to pineapple and is a key ingredient used as flavor enhancer in processed orange juices. Hence, the statement “ethyl butanoate is a liquid at room temperature” is true.
(h)
Interpretation:
The statement “ethyl butanoate is a water soluble” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(h)
Answer to Problem 4MCP
The given statement is false because ethyl butanoate is insoluble in water.
Explanation of Solution
Ethyl butanoate is very hydrophobic molecule and it is insoluble in water.
Ethyl butanoate forces themselves between water molecules; they break the strong hydrogen bonds between water molecules without replacing them. This makes the process energetically less profitable and so solubility decreases. As the chain lengths increase, the hydrocarbon parts of the ester molecules start to get in the way.
Hence, the statement “ethyl butanoate is a water soluble” is false because it insoluble in water due to its hydrophobic nature.
(i)
Interpretation:
The statement “Ethyl butanoate has a carboxyl
(i)
Answer to Problem 4MCP
The given statement is false because ethyl butanoate has a carbonyl functional group.
Explanation of Solution
Esters are carboxylic acid derivatives. The carbonyl group of the ester is polar and can participate in dipole-dipole attractions.
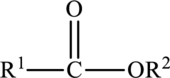
In an ester, the second oxygen atom is bonded to another carbon atom.
A carboxyl group consists of both polar carbonyl and hydroxyl functional groups.
The structure of ethyl butanoate is,
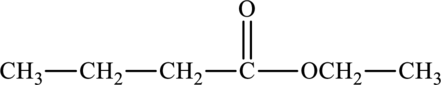
Ethyl butanoate shows the presence of carbonyl group and not carboxyl group.
Hence, the statement “Ethyl butanoate has a carboxyl functional group” is false because it consists of polar carbonyl group.
(j)
Interpretation:
The statement “ethyl butanoate is a structural isomer of hexanoic acid” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(j)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
Structural isomers are those with same molecular formula but differ in arrangement of atoms.
The molecular formula of ethyl butanoate is
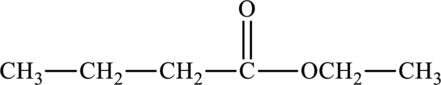
The molecular formula of hexanoic acid is
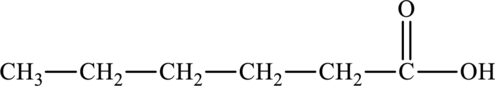
Both these are structural isomers with each other and hence, the statement “ethyl butanoate is a structural isomer of hexanoic acid” is true.
Want to see more full solutions like this?
Chapter 14 Solutions
General, Organic, and Biochemistry
- The statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant. In each table, there may be one statement that is faise because it contradicts the other three statements. If you find a false statement, check the box next to t Otherwise, check the "no false statements" box under the table. statement false? AG"1 no false statements: statement false? AG-0 0 InK-0 0 K-1 0 AH-TAS no false statements 2arrow_forwardComplete the following esterification reactions by drawing the line formulas of the carboxylic acid and alcohol required to form the ester shown. catalyst catalyst catalyst apricot fragrancearrow_forwardShow the saponification products of the following ester: You don't need to draw in the Na+ cation. catalyst, A catalyst, A catalyst, Aarrow_forward
- What would happen if the carboxylic acid and alcohol groups were on the same molecule? In essence, the molecule reacts with itself. Draw the structure of the products formed in this manner using the reactants below. If two functional groups interact with one another on the same molecule, this is called an “intramolecular" (within one) rather than "intermolecular" (between two or more) attack. OH OH catalyst OH HO catalyst catalyst HO OHarrow_forwardQ3: Write in the starting alkyl bromide used to form the following products. Include any reactants, reagents, and solvents over the reaction arrow. If more than one step is required, denote separate steps by using 1), 2), 3), etc. H OH racemic OH OH 5 racemicarrow_forwardDraw the Lewis structure of the SO3-O(CH3)2 complex shown in the bottom right of slide 2in lecture 3-3 (“Me” means a CH3 group) – include all valence electron pairs and formal charges.From this structure, should the complex be a stable molecule? Explain.arrow_forward
- please add appropriate arrows, and tell me clearly where to add arrows, or draw itarrow_forwardWhat I Have Learned Directions: Given the following reaction and the stress applied in each reaction, answer the question below. A. H2(g) + Cl2(g) 2 HCl(g) Stress applied: Decreasing the pressure 1. What is the Keq expression? 2. What will be the effect in the number of moles of HCl(g)? 3. What will be the Equilibrium Shift or the reaction? B. Fe3O4(s) + 4 H2(g) + heat 53 Fe(s) + 4 H₂O(g) Stress applied: Increasing the temperature 1. What is the Keq expression?. 2. What will be the effect in the volume of water vapor collected? 3. What will be the Equilibrium Shift or the reaction? C. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) + heat Stress applied: Increasing the volume of the container 1. What is the Keq expression?. 2. What will be the effect in the amount of H₂O? 3. What will be the Equilibrium Shift or the reaction?arrow_forwardConsider the solubility products (Ksp values) for the following compounds:SrSO4 (Ksp = 7.6 x 10−7), BaSO4 (Ksp = 1.5 x 10−9), SrCO3 (Ksp = 7.0 x 10−10), BaCO3 (Ksp = 1.6 x 10−9)Which anion is the harder base, CO32− or SO42−? Justify your answer.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





