
Concept explainers
(a)
Interpretation: The Lewis structure of Otezla needs to be completed.
Concept Introduction:
Lewis structure shows the arrangement of total number of valence electrons in a molecule. The electrons involved in bonding are known as bonding electrons and these are represented as bonds or line between two atoms (bonded together). On the other hand, electrons which are not involved in the bonding are known as lone pair of electrons. They are represented as dots (in pair) on symbol of an atom.
(a)
Explanation of Solution
The given structure is as follows:
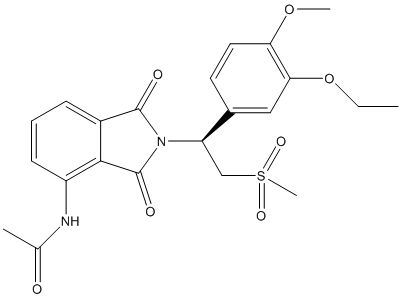
Here, C, N, O and S atoms are present. The number of valence electrons of C, N, O and S atom is 4, 5, 6 and 6 respectively.
In the given molecule, C atom will form 4 covalent bonds so all its electrons will be represented as bond pairs, O atom will form 2 lone pair and 2 bond pair of electrons, S atom will have all the valence electrons involved in bonding and N atom will have 1 lone pair of electrons and 3 bond pairs.
The distribution of valence electrons is represented as follows:
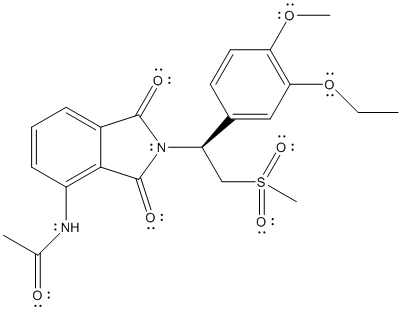
Here, all the oxygen atoms will have 2 lone pair of electrons, N atom will have 1 lone pair of electrons and there will be no valence electrons on S atom.
(b)
Interpretation:In the structure of Otezla, the atom which is exception to octet rule needs to be identified.
Concept Introduction:
In the Lewis structure of a molecule, total number of valence electrons are distributed such that all the atoms have complete octets. In complete octet, atoms have 8 valance electrons in their outermost shell (except H which has 2 electrons).
(b)
Explanation of Solution
The complete Lewis structure of Otezla is as follows:
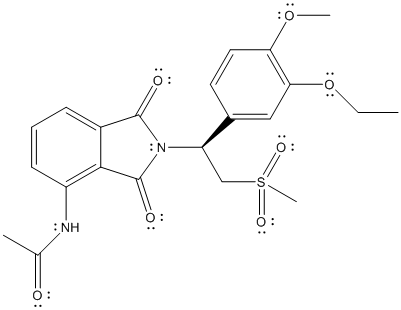
Here, all the valence electrons of C atoms are involved in bonding, oxygen atom has 2 lone pair of electrons and 2 bond pairs, N atom has 1 lone pair of electrons and 3 bond pairs. Thus, C, O and N atoms have complete octet. The only atom with exception to the octet rule is S. The total number of valence electrons on S atom is 6 thus, it can share 2 electrons with other atoms to complete the octet like O atom but, here S is involved in 2 double bonds with 2 O atoms and 2 single bonds with 2 C atom. The total number of electrons involved will be 12 which is exception to octet rule.
(c)
Interpretation: The valence-shell electron-pair repulsion model needs to be used to predict all the bond angles in Otezla.
Concept Introduction:
The valence-shell electron pair repulsion model is used to determine the hybridization, geometry and bond angles of central atoms in given structure.
To determine the hybridization, total number of bond pairs and lone pair of electrons are determined for a central atom. Depending on the total number of electron pair, hybridization is decided. Each hybridization corresponds to a geometry and respective bond angle.
(c)
Explanation of Solution
The complete Lewis structure of Otezla is as follows:
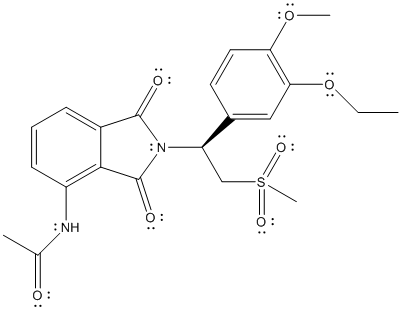 T
T
Here, the C atom with 4 single bonds is
All the bond angles are represented in Lewis structure as follows:
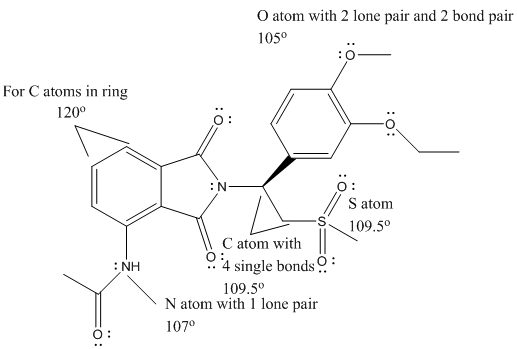
(d)
Interpretation: Whether Otezla is polar or non-polar needs to be explained.
Concept Introduction: In a molecule, if there is difference in electronegativity between atoms then it is said to be polar in nature. In other way, if polar bonds are present in the molecule, then it is considered as a polar molecule.
(d)
Explanation of Solution
A molecule is said to be polar if there are polar bonds present in it. A bond is said to be polar if there is difference in electronegativity value of two bonded atoms.
The given structure is as follows:
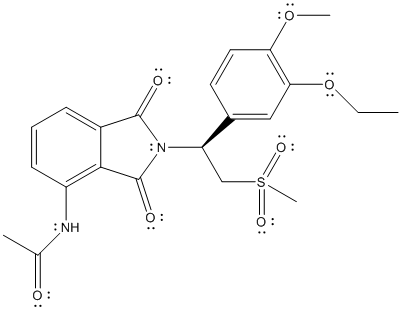
There is no electronegativity difference between C and H atom. The electronegative atoms in the above molecule are N, O and S atom.
Thus, N-C, N-H, C-O and C-S are polar bonds and molecule will be polar in nature.
(e)
Interpretation: The two resonance structures of Otezla needs to be drawn.
Concept Introduction: The resonance structures are possible in a molecule if there are lone pair of electrons and alternate pi bonds are present.
(e)
Explanation of Solution
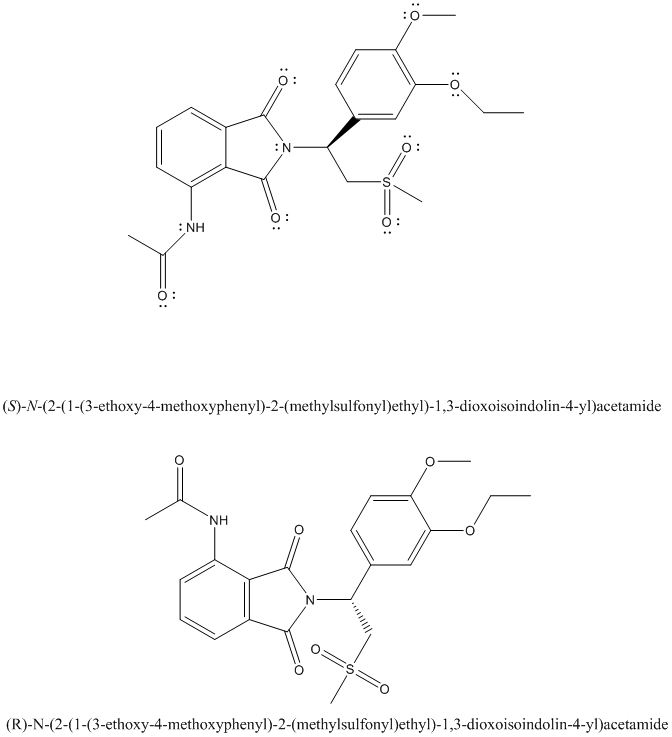
The complete Lewis structure of Otezla is as follows:
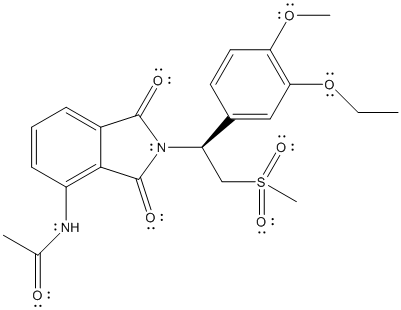
Two resonance structures are as follows:
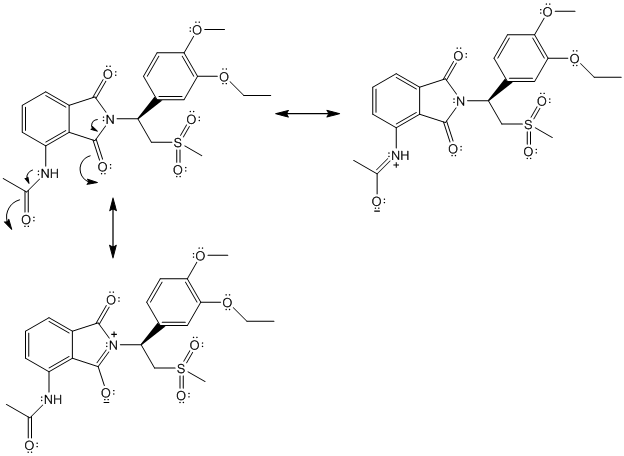
(f)
Interpretation: The molecular formula of Otezla needs to be determined.
Concept Introduction: The molecular formula of a molecule can be determined from its structure. Total number of atoms of same atoms can be determined from the structure and formula can be determined.
(f)
Explanation of Solution
The complete Lewis structure of Otezla is as follows:
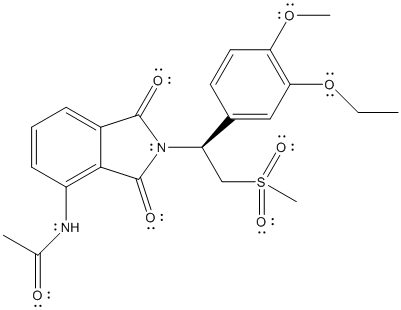
In the above structure, there are C, H, N, O and S atoms. By calculating the total number of atoms in the molecule, molecular formula can be calculated.
The molecular formula of the molecule will be
(g)
Interpretation: The intermolecular forces present between Otezla molecules needs to be determined.
Concept Introduction: The intermolecular forces are type of interactions between the molecules. Non-polar molecules only have London dispersion forces and polar molecules can have other interactions as well like hydrogen bonding, dipole-dipole interactions, Van der Waals forces etc.
(g)
Explanation of Solution
The complete Lewis structure of Otezla is as follows:
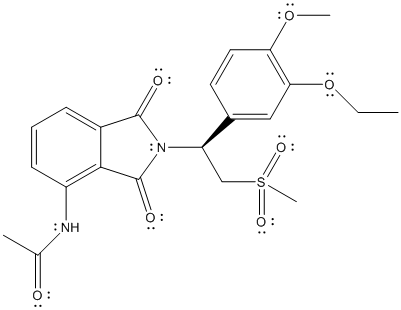
Due to the presence of non-polar bonds, London dispersion forces are present in the molecules. Also due to N-H bond, hydrogen bonding is possible between the molecules.
(h)
Interpretation: The stereocenter of Otezla needs to be identified as R or S.
Concept Introduction:
The Cahn-Ingold-Prelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations 'R ' (from the Latin rectus, meaning right-handed) or ' S ' (from the Latin sinister, meaning left-handed).
In the first step, priority is given to 4 different groups attached to the stereocenter. The direction of 4th priority group should be away from the observer. In the second step, direction from 1 to 3 priority group is determined. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
(h)
Explanation of Solution
The complete Lewis structure is represented as follows:
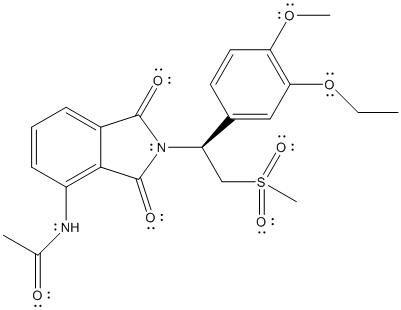
The stereocenter is point where 4 different substituents are present on a carbon atom. Molecules with stereocenter are known as chiral molecules.
The chiral center is labelled as follows:
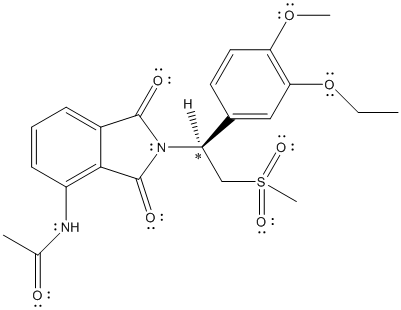
The numbering according to priority rules will be:
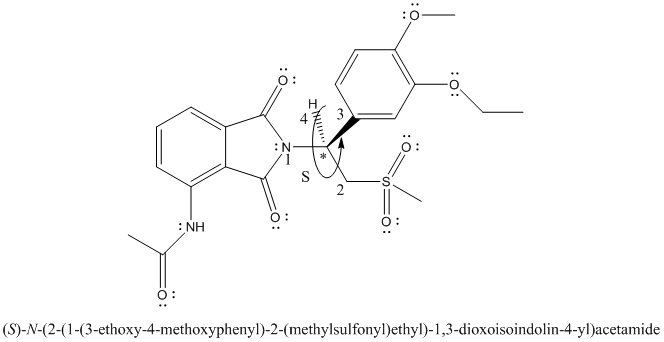
The direction from 1 to 3 priority group is anticlockwise thus, the configuration is S.
(i)
Interpretation: The enantiomer of Otezla needs to be drawn.
Concept Introduction:
The chirality in a group in a molecule results in the formation of two enantiomers. These enantiomers are non-superimposable mirror image of each other.
(i)
Explanation of Solution
The enantiomer of Otezla will be R configuration and it is represented as follows:
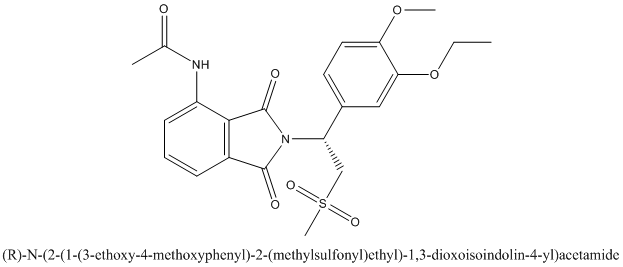
Want to see more full solutions like this?
Chapter 14 Solutions
Introduction To General, Organic, And Biochemistry
- Q2: We would not expect the following primary alkyl halide to go through an SN1 reaction. However, it can go through an SN1 mechanism. Explain why. Hint: Think about what happens when the leaving group leaves. CI NaO EtOH H བྱིས་ Harrow_forwardI performed this experiment, but I'm so confused. How do I find the first two blank columns using the data provided. What is the [I^-] mol/L and [S2O8^-2] mol/L. How do I find this? Please help!arrow_forwardExample 3 A molecule is achiral if it has a plane of symmetry in any conformation. The given conformation of 2,3-dibromobutane below does not have a plane of symmetry. Will rotation around the C2-C3 bond form a conformation with a plane of symmetry? Draw the conformation to find out. DIY: Do the same for: H3C Brill rotate H CH3 OH HO Brarrow_forward
- 120 100 20 20 bound drug/free drug (%) 60 40 60 80 80 0 0 Scatchard Plot of Drug Binding 20 20 40 60 80 100 120 bound drug (nM)arrow_forwardUsing diethylmalonate and benzyl bromide as your only as your only source of carbon, propose a synthesis for the following compound.arrow_forwardplease helparrow_forward
- What is the difference between (+)-(S)-methamphetamine and (-)-(R)-methamphetamine versus levo-methamphetamine and dextro-methamphetamine, D-methamphetamine, and L-methamphetamine, and N-methamphetamine? Please use scholarly sources and in-text citations.arrow_forwardanswer all the questions with explanationarrow_forwardPlease draw a mechanism don't write sentarrow_forward
- From this COZY spectrum, how do you know which protons are next to each other?arrow_forward5. A buffer consists of 0.45 M NH, and 0.25 M NH-CI (PK of NH 474) Calculate the pH of the butter. Ans: 9.52 BAS PH-9.26 +10g (10.95)) 14-4.59 PH=4.52 6. To 500 ml of the buffer on #5 a 0.20 g of sample of NaOH was added a Write the net ionic equation for the reaction which occurs b. Should the pH of the solution increase or decrease sightly? Calculate the pH of the buffer after the addition Ans: 9.54arrow_forwardExplain the inductive effect (+I and -I) in benzene derivatives.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





