
Introduction To General, Organic, And Biochemistry
12th Edition
ISBN: 9781337571357
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 39P
15-43 Triamcinolone acetonide, the active ingredient in Azmacort Inhalation Aerosol, is a steroid used to treat bronchial asthma.
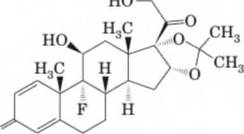
Triamcinolone acetonide
- Label the eight stereocenters in this molecule.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please correct answer and don't use hand rating and don't use Ai solution
How to determine if this is N- ethylsaccharin or O-ethylsaccharin or a mixture of both based on chemical shifts.
None
Chapter 14 Solutions
Introduction To General, Organic, And Biochemistry
Ch. 14.1 - Prob. 14.1QCCh. 14.2 - Problem 15-2 Assign priorities to the groups in...Ch. 14.2 - Problem 15-3 Assign an R or S configuration to the...Ch. 14.3 - Problem 15-4 3-Amino-2-butanol has two...Ch. 14.3 - Prob. 14.5QCCh. 14.3 - Prob. 14.6QCCh. 14 - 15-7 Answer true or false. The cis and trans...Ch. 14 - 15-8 What does the term “chiral” mean? Give an...Ch. 14 - 15-9 What does the term “achiral” mean? Give an...Ch. 14 - 15-10 Define the term “stereoisomer.” Name three...
Ch. 14 - 15-11 In what way are constitutional isomers...Ch. 14 - 15-12 Which of the following objects are chiral...Ch. 14 - Prob. 7PCh. 14 - Prob. 8PCh. 14 - Prob. 9PCh. 14 - Prob. 10PCh. 14 - 15-15 Explain why the carbon of a carbonyl group...Ch. 14 - 15-16 Which of the following compounds contain...Ch. 14 - 15-17 Which of the following compounds contain...Ch. 14 - Prob. 14PCh. 14 - 15-19 Draw the mirror image for each molecule: OH...Ch. 14 - Prob. 16PCh. 14 - 15-21 Answer true or false. For a molecule with...Ch. 14 - Prob. 18PCh. 14 - Prob. 19PCh. 14 - Prob. 20PCh. 14 - Prob. 21PCh. 14 - 15-26 For centuries, Chinese herbal medicine has...Ch. 14 - Prob. 23PCh. 14 - Prob. 24PCh. 14 - Prob. 25PCh. 14 - Prob. 26PCh. 14 - Prob. 27PCh. 14 - Prob. 28PCh. 14 - Prob. 29PCh. 14 - Prob. 30PCh. 14 - 15-35 Following are structural formulas for three...Ch. 14 - Prob. 32PCh. 14 - 15-37 Consider a cyclohexane ring substituted with...Ch. 14 - Prob. 34PCh. 14 - Prob. 35PCh. 14 - Prob. 36PCh. 14 - 15-41 Compound A(C5Hh, is not optically active and...Ch. 14 - Prob. 38PCh. 14 - 15-43 Triamcinolone acetonide, the active...Ch. 14 - 15-44 Consider the structure of the...Ch. 14 - Prob. 41PCh. 14 - 15-46 Consider Lunesta, a nonbenzodiazepine...Ch. 14 - Prob. 43PCh. 14 - Prob. 44P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3) Propagation of uncertainty. Every measurement has uncertainty. In this problem, we'll evaluate the uncertainty in every step of a titration of potassium hydrogen phthalate (a common acid used in titrations, abbreviated KHP, formula CsH5KO4) with NaOH of an unknown concentration. The calculation that ultimately needs to be carried out is: concentration NaOH 1000 x mass KHP × purity KHP molar mass KHP x volume NaOH Measurements: a) You use a balance to weigh 0.3992 g of KHP. The uncertainty is ±0.15 mg (0.00015 g). b) You use a buret to slowly add NaOH to the KHP until it reaches the endpoint. It takes 18.73 mL of NaOH. The uncertainty of the burst is 0.03 mL.. c) The manufacturer states the purity of KHP is 100%±0.05%. d) Even though we don't think much about them, molar masses have uncertainty as well. The uncertainty comes from the distribution of isotopes, rather than random measurement error. The uncertainty in the elements composing KHP are: a. Carbon: b. Hydrogen: ±0.0008…arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardHow would you use infrared spectroscopy to distinguish between the following pairs of constitutional isomers? (a) CH3C=CCH3 || and CH3CH2C=CH (b) CH3CCH=CHCH3 and CH3CCH2CH=CH2 Problem 12-41 The mass spectrum (a) and the infrared spectrum (b) of an unknown hydrocarbon are shown. Propose as many structures as you can. (a) 100 Relative abundance (%) 80 60 60 40 200 20 (b) 100 Transmittance (%) 10 20 20 80- 60- 40- 20 40 60 80 100 120 140 m/z 500 4000 3500 3000 2500 2000 1500 Wavenumber (cm-1) 1000arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY