
Introduction To General, Organic, And Biochemistry
12th Edition
ISBN: 9781337571357
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 42P
15-46 Consider Lunesta, a nonbenzodiazepine hypnotic agent (i.e., sleep-inducing drug) that is frequently advertised on TV commercials. Answer the following questions with respect to the given structure:
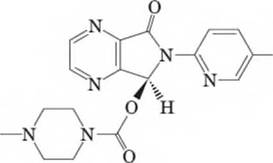
Lunesta
- Determine the molecular formula for Lunesta.
stereocenter(s) and therefore
possible stereoisomer(s). Of the possible
stereocenter(s),
is/are R and
is/are S.
- Does Lunesta have an enantiomer? Does it have a diastereomer?
- Which of the following is true about an enantiomer of Lunesta? Identify all that apply:
Draw an enantiomer of Lunesta.
Examine the derivative of the representation of the six-membered ring found in Lunesta. Draw the alternative chair conformations of this ring and label the more stable chair conformation. (Chapter 11)
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Synthesize the following:
Did you report your data to the correct number of significant
figures?
Temperature of cold water (°C)
4.0
Temperature of hot water ("C)
87.0
Volume of cold water (mL)
94.0
Volume of hot water (mL)
78.0
Final temperature after mixing ("C)
41.0
Mass of cold water (g)
94.0
Mass of hot water (g)
78.0
Calorimeter constant (J/°C)
12.44
How to calculate the calorimeter constant
please draw the arrows
Chapter 14 Solutions
Introduction To General, Organic, And Biochemistry
Ch. 14.1 - Prob. 14.1QCCh. 14.2 - Problem 15-2 Assign priorities to the groups in...Ch. 14.2 - Problem 15-3 Assign an R or S configuration to the...Ch. 14.3 - Problem 15-4 3-Amino-2-butanol has two...Ch. 14.3 - Prob. 14.5QCCh. 14.3 - Prob. 14.6QCCh. 14 - 15-7 Answer true or false. The cis and trans...Ch. 14 - 15-8 What does the term “chiral” mean? Give an...Ch. 14 - 15-9 What does the term “achiral” mean? Give an...Ch. 14 - 15-10 Define the term “stereoisomer.” Name three...
Ch. 14 - 15-11 In what way are constitutional isomers...Ch. 14 - 15-12 Which of the following objects are chiral...Ch. 14 - Prob. 7PCh. 14 - Prob. 8PCh. 14 - Prob. 9PCh. 14 - Prob. 10PCh. 14 - 15-15 Explain why the carbon of a carbonyl group...Ch. 14 - 15-16 Which of the following compounds contain...Ch. 14 - 15-17 Which of the following compounds contain...Ch. 14 - Prob. 14PCh. 14 - 15-19 Draw the mirror image for each molecule: OH...Ch. 14 - Prob. 16PCh. 14 - 15-21 Answer true or false. For a molecule with...Ch. 14 - Prob. 18PCh. 14 - Prob. 19PCh. 14 - Prob. 20PCh. 14 - Prob. 21PCh. 14 - 15-26 For centuries, Chinese herbal medicine has...Ch. 14 - Prob. 23PCh. 14 - Prob. 24PCh. 14 - Prob. 25PCh. 14 - Prob. 26PCh. 14 - Prob. 27PCh. 14 - Prob. 28PCh. 14 - Prob. 29PCh. 14 - Prob. 30PCh. 14 - 15-35 Following are structural formulas for three...Ch. 14 - Prob. 32PCh. 14 - 15-37 Consider a cyclohexane ring substituted with...Ch. 14 - Prob. 34PCh. 14 - Prob. 35PCh. 14 - Prob. 36PCh. 14 - 15-41 Compound A(C5Hh, is not optically active and...Ch. 14 - Prob. 38PCh. 14 - 15-43 Triamcinolone acetonide, the active...Ch. 14 - 15-44 Consider the structure of the...Ch. 14 - Prob. 41PCh. 14 - 15-46 Consider Lunesta, a nonbenzodiazepine...Ch. 14 - Prob. 43PCh. 14 - Prob. 44P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardcan you please answer both these questions and draw the neccesaryarrow_forwardcan you please give the answer for both these pictures. thankyouarrow_forward
- Part 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) | Bakelite like polymer Using: Resorcinol + NaOH + Formalinarrow_forwardQuestion 19 0/2 pts 3 Details You have a mixture of sodium chloride (NaCl) and potassium chloride (KCl) dissolved in water and want to separate out the Cl- ions by precipitating them out using silver ions (Ag+). The chemical equation for the net ionic reaction of NaCl and KCl with silver nitrate, AgNO3, is shown below. Ag+(aq) + Cl(aq) → AgCl(s) The total mass of the NaCl/KCl mixture is 1.299 g. Adding 50.42 mL of 0.381 M solution precipitates out all of the Cl-. What are the masses of NaCl and KCl in the mixture? Atomic masses: g: Mass of NaCl g: Mass of KCL Ag = 107.868 g mol- 1 Cl = 35.453 g mol- 1 K = 39.098 g mol- N = 14.007 g mol−1 Na = 22.99 g mol−1 0 = 15.999 g mol 1 Question Help: ✓ Message instructor Submit Questionarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerolarrow_forward
- Identify the missing starting materials/ reagents/ products in the following reactions. Show the stereochemistry clearly in the structures, if any. If there is a major product, draw the structures of the major product with stereochemistry clearly indicated where applicable. Show only the diastereomers (you do not have to draw the pairs of enantiomers). If you believe that multiple products are formed in approximately equal amounts (hence neither is the major product), draw the structures of the products, and show the detailed mechanism of these reactions to justify the formation of the multiple products. If you believe no product is formed, explain why briefly. (6 mark for each, except f and g, which are 10 mark each)arrow_forward3. What starting material would you use to synthesize 3-hydroxypentanoic acid using a NaBH4 reduction?arrow_forward1. Give stereochemical (Fischer projection) formulas for all (but no extras) the stereoisomers that could theoretically form during the reduction of a. the carbonyl group of 2-methyl-3--pentanone b. both carbonyl groups of 2,4-pentanedione (careful!) 2. Predict the products of the reduction of O=CCH2CH2CH2C=O with a. LiAlH4 b. NaBH4 CH3 OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY