
Concept explainers
Interpretation:
The energies of rotation for ammonia,
Concept introduction:
Atoms of a molecule rotate in space about its moment of inertia. The rotational quantum number is represented by the symbol
Answer to Problem 14.98E
The energies of rotation for ammonia,
| 1 | -1 | 3753.185 |
| 1 | 0 | 1264.06 |
| 1 | 1 | 3753.185 |
| 2 | -2 | 13748.68 |
| 2 | -1 | 6281.305 |
| 2 | 0 | 3792.18 |
| 2 | 1 | 6281.305 |
| 2 | 2 | 13748.68 |
| 3 | -3 | 29986.49 |
| 3 | -2 | 17540.86 |
| 3 | -1 | 10073.49 |
| 3 | 0 | 7584.36 |
| 3 | 1 | 10073.49 |
| 3 | 2 | 17540.86 |
| 3 | 3 | 29986.49 |
| 4 | -4 | 52466.6 |
| 4 | -3 | 35042.73 |
| 4 | -2 | 22597.1 |
| 4 | -1 | 15129.73 |
| 4 | 0 | 12640.6 |
| 4 | 1 | 15129.73 |
| 4 | 2 | 22597.1 |
| 4 | 3 | 52466.6 |
| 4 | 4 | 52466.6 |
| 5 | -5 | 81189.03 |
| 5 | -4 | 58786.9 |
| 5 | -3 | 41363.03 |
| 5 | -2 | 28917.4 |
| 5 | -1 | 21450.03 |
| 5 | 0 | 18960.9 |
| 5 | 1 | 21450.03 |
| 5 | 2 | 28917.4 |
| 5 | 3 | 41363.03 |
| 5 | 4 | 58786.9 |
| 5 | 5 | 81189.03 |
| 6 | -6 | 116153.8 |
| 6 | -5 | 88773.39 |
| 6 | -4 | 66371.26 |
| 6 | -3 | 48947.39 |
| 6 | -2 | 36501.76 |
| 6 | -1 | 29034.39 |
| 6 | 0 | 26545.26 |
| 6 | 1 | 29034.39 |
| 6 | 2 | 36501.76 |
| 6 | 3 | 48947.39 |
| 6 | 4 | 66371.26 |
| 6 | 5 | 88773.39 |
| 6 | 6 | 116153.8 |
| 7 | -7 | 157360.8 |
| 7 | -6 | 125002.2 |
| 7 | -5 | 97621.81 |
| 7 | -4 | 75219.68 |
| 7 | -3 | 57795.81 |
| 7 | -2 | 45350.18 |
| 7 | -1 | 37882.81 |
| 7 | 0 | 35393.68 |
| 7 | 1 | 37882.81 |
| 7 | 2 | 45350.18 |
| 7 | 3 | 57795.81 |
| 7 | 4 | 75219.68 |
| 7 | 5 | 97621.81 |
| 7 | 6 | 125002.2 |
| 7 | 7 | 157360.8 |
| 8 | -8 | 204810.2 |
| 8 | -7 | 167473.3 |
| 8 | -6 | 135114.7 |
| 8 | -5 | 107734.3 |
| 8 | -4 | 85332.16 |
| 8 | -3 | 67908.29 |
| 8 | -2 | 55462.66 |
| 8 | -1 | 47995.29 |
| 8 | 0 | 45506.16 |
| 8 | 1 | 47995.29 |
| 8 | 2 | 55462.66 |
| 8 | 3 | 67908.29 |
| 8 | 4 | 85332.16 |
| 8 | 5 | 107734.3 |
| 8 | 6 | 135114.7 |
| 8 | 7 | 167473.3 |
| 8 | 8 | 204810.2 |
| 9 | -9 | 258501.8 |
| 9 | -8 | 216186.7 |
| 9 | -7 | 178849.8 |
| 9 | -6 | 146491.2 |
| 9 | -5 | 119110.8 |
| 9 | -4 | 96708.7 |
| 9 | -3 | 79284.83 |
| 9 | -2 | 66839.2 |
| 9 | -1 | 59371.83 |
| 9 | 0 | 56882.7 |
| 9 | 1 | 59371.83 |
| 9 | 2 | 66839.2 |
| 9 | 3 | 79284.83 |
| 9 | 4 | 96708.7 |
| 9 | 5 | 119110.8 |
| 9 | 6 | 146491.2 |
| 9 | 7 | 178849.8 |
| 9 | 8 | 216186.7 |
| 9 | 9 | 258501.8 |
| 10 | -10 | 318435.8 |
| 10 | -9 | 271142.4 |
| 10 | -8 | 228827.3 |
| 10 | -7 | 191490.4 |
| 10 | -6 | 159131.8 |
| 10 | -5 | 131751.4 |
| 10 | -4 | 109349.3 |
| 10 | -3 | 91925.43 |
| 10 | -2 | 79479.8 |
| 10 | -1 | 72012.43 |
| 10 | 0 | 69523.3 |
| 10 | 1 | 72012.43 |
| 10 | 2 | 79479.8 |
| 10 | 3 | 91925.43 |
| 10 | 4 | 109349.3 |
| 10 | 5 | 131751.4 |
| 10 | 6 | 159131.8 |
| 10 | 7 | 191490.4 |
| 10 | 8 | 228827.3 |
| 10 | 9 | 271142.4 |
| 10 | 10 | 318435.8 |
For the rotational quantum number
For the rotational quantum number
For the rotational quantum number
For the rotational quantum number
For the rotational quantum number
For the rotational quantum number
For the rotational quantum number
For the rotational quantum number
For the rotational quantum number
For the rotational quantum number
The energy level diagram for all the rotational levels is shown below.
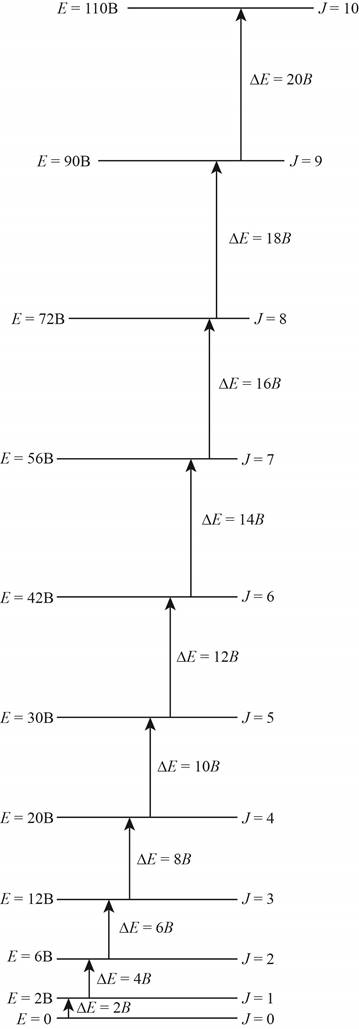
Explanation of Solution
The formula to energy of rotation (
Where,
•
•
The formula for
The formula for
Where,
•
•
The value of
Substitute the value of
The value of
Substitute the value of
The value of
The degeneracy is calculated by the formula given below.
For the rotational quantum number
The value of
The value of
Substitute the value of
Therefore, the degeneracy is
Substitute the value of
Similarly the value of
| 1 | -1 | 3753.185 |
| 1 | 0 | 1264.06 |
| 1 | 1 | 3753.185 |
For the rotational quantum number
Substitute the value of
Therefore, the degeneracy is
Similarly the value of
| 2 | -2 | 13748.68 |
| 2 | -1 | 6281.305 |
| 2 | 0 | 3792.18 |
| 2 | 1 | 6281.305 |
| 2 | 2 | 13748.68 |
For the rotational quantum number
Substitute the value of
Therefore, the degeneracy is
Similarly the value of
| 3 | -3 | 29986.49 |
| 3 | -2 | 17540.86 |
| 3 | -1 | 10073.49 |
| 3 | 0 | 7584.36 |
| 3 | 1 | 10073.49 |
| 3 | 2 | 17540.86 |
| 3 | 3 | 29986.49 |
For the rotational quantum number
Substitute the value of
Therefore, the degeneracy is
Similarly the value of
| 4 | -4 | 52466.6 |
| 4 | -3 | 35042.73 |
| 4 | -2 | 22597.1 |
| 4 | -1 | 15129.73 |
| 4 | 0 | 12640.6 |
| 4 | 1 | 15129.73 |
| 4 | 2 | 22597.1 |
| 4 | 3 | 52466.6 |
| 4 | 4 | 52466.6 |
For the rotational quantum number
Substitute the value of
Therefore, the degeneracy is
Similarly the value of
| 5 | -5 | 81189.03 |
| 5 | -4 | 58786.9 |
| 5 | -3 | 41363.03 |
| 5 | -2 | 28917.4 |
| 5 | -1 | 21450.03 |
| 5 | 0 | 18960.9 |
| 5 | 1 | 21450.03 |
| 5 | 2 | 28917.4 |
| 5 | 3 | 41363.03 |
| 5 | 4 | 58786.9 |
| 5 | 5 | 81189.03 |
For the rotational quantum number
Substitute the value of
Therefore, the degeneracy is
Similarly the value of
| 6 | -6 | 116153.8 |
| 6 | -5 | 88773.39 |
| 6 | -4 | 66371.26 |
| 6 | -3 | 48947.39 |
| 6 | -2 | 36501.76 |
| 6 | -1 | 29034.39 |
| 6 | 0 | 26545.26 |
| 6 | 1 | 29034.39 |
| 6 | 2 | 36501.76 |
| 6 | 3 | 48947.39 |
| 6 | 4 | 66371.26 |
| 6 | 5 | 88773.39 |
| 6 | 6 | 116153.8 |
For the rotational quantum number
Substitute the value of
Therefore, the degeneracy is
Similarly the value of
| 7 | -7 | 157360.8 |
| 7 | -6 | 125002.2 |
| 7 | -5 | 97621.81 |
| 7 | -4 | 75219.68 |
| 7 | -3 | 57795.81 |
| 7 | -2 | 45350.18 |
| 7 | -1 | 37882.81 |
| 7 | 0 | 35393.68 |
| 7 | 1 | 37882.81 |
| 7 | 2 | 45350.18 |
| 7 | 3 | 57795.81 |
| 7 | 4 | 75219.68 |
| 7 | 5 | 97621.81 |
| 7 | 6 | 125002.2 |
| 7 | 7 | 157360.8 |
For the rotational quantum number
Substitute the value of
Therefore, the degeneracy is
Similarly the value of
| 8 | -8 | 204810.2 |
| 8 | -7 | 167473.3 |
| 8 | -6 | 135114.7 |
| 8 | -5 | 107734.3 |
| 8 | -4 | 85332.16 |
| 8 | -3 | 67908.29 |
| 8 | -2 | 55462.66 |
| 8 | -1 | 47995.29 |
| 8 | 0 | 45506.16 |
| 8 | 1 | 47995.29 |
| 8 | 2 | 55462.66 |
| 8 | 3 | 67908.29 |
| 8 | 4 | 85332.16 |
| 8 | 5 | 107734.3 |
| 8 | 6 | 135114.7 |
| 8 | 7 | 167473.3 |
| 8 | 8 | 204810.2 |
For the rotational quantum number
Substitute the value of
Therefore, the degeneracy is
Similarly the value of
| 9 | -9 | 258501.8 |
| 9 | -8 | 216186.7 |
| 9 | -7 | 178849.8 |
| 9 | -6 | 146491.2 |
| 9 | -5 | 119110.8 |
| 9 | -4 | 96708.7 |
| 9 | -3 | 79284.83 |
| 9 | -2 | 66839.2 |
| 9 | -1 | 59371.83 |
| 9 | 0 | 56882.7 |
| 9 | 1 | 59371.83 |
| 9 | 2 | 66839.2 |
| 9 | 3 | 79284.83 |
| 9 | 4 | 96708.7 |
| 9 | 5 | 119110.8 |
| 9 | 6 | 146491.2 |
| 9 | 7 | 178849.8 |
| 9 | 8 | 216186.7 |
| 9 | 9 | 258501.8 |
For the rotational quantum number
Substitute the value of
Therefore, the degeneracy is
Similarly the value of
| 10 | -10 | 318435.8 |
| 10 | -9 | 271142.4 |
| 10 | -8 | 228827.3 |
| 10 | -7 | 191490.4 |
| 10 | -6 | 159131.8 |
| 10 | -5 | 131751.4 |
| 10 | -4 | 109349.3 |
| 10 | -3 | 91925.43 |
| 10 | -2 | 79479.8 |
| 10 | -1 | 72012.43 |
| 10 | 0 | 69523.3 |
| 10 | 1 | 72012.43 |
| 10 | 2 | 79479.8 |
| 10 | 3 | 91925.43 |
| 10 | 4 | 109349.3 |
| 10 | 5 | 131751.4 |
| 10 | 6 | 159131.8 |
| 10 | 7 | 191490.4 |
| 10 | 8 | 228827.3 |
| 10 | 9 | 271142.4 |
| 10 | 10 | 318435.8 |
The energy level diagram for all the rotational levels is shown below.
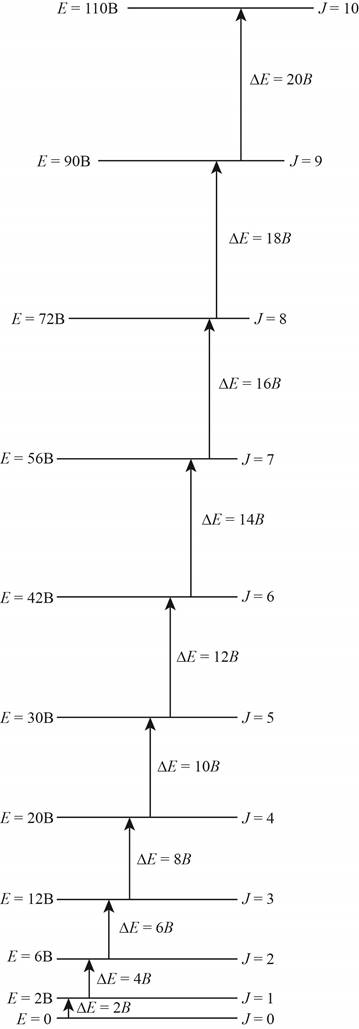
Figure 1
The energies of rotation for ammonia,
Want to see more full solutions like this?
Chapter 14 Solutions
Bundle: Physical Chemistry, 2nd + Student Solutions Manual
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

