
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.68P
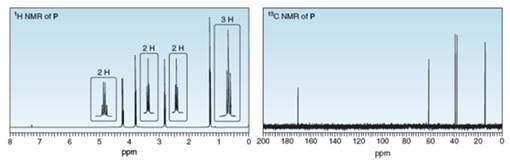
Compound P has molecular formula
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing
150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3
Ag+ + S20
Ag(S203)¯
K₁ = 7.4 × 108
Ag(S203)¯ + S20¯ = Ag(S203)
K₂ = 3.9 x 104
ΗΝ,
cyclohexanone
pH 4-5
Draw Enamine
I
I
CH3CH2Br
THF, reflux
H3O+
I
Drawing
Draw Iminium Ion
:0: :0:
Select to Add Arrows
:0:
(CH3)2NH
:0:
■ Select to Add Arrows
:0:
:0:
(CH3)2NH
■ Select to Add Arrows
Chapter 14 Solutions
ORGANIC CHEMISTRY
Ch. 14 - Problem 14.1 The NMR spectrum of recorded on a ...Ch. 14 - Prob. 14.2PCh. 14 - How many NMR signals does each compound show?
a....Ch. 14 - How many 1H NMR signals does each...Ch. 14 - How many 1H NMR signals does each compound give?...Ch. 14 - Label the protons in each highlighted CH2 group as...Ch. 14 - How many 1H NMR signals would you expect for each...Ch. 14 - Prob. 14.8PCh. 14 - For each compound, first label each different type...Ch. 14 - Label each statement as True or False. a. When a...
Ch. 14 - Prob. 14.11PCh. 14 - Problem 14.12 Which compound give a NMR spectrum...Ch. 14 - Prob. 14.13PCh. 14 - Prob. 14.14PCh. 14 - For each compound give the number of 1H NMR...Ch. 14 - Prob. 14.16PCh. 14 - Prob. 14.17PCh. 14 - Problem 14.18 Describe the NMR spectrum of each...Ch. 14 - Problem 14.19 Draw a splitting diagram for in ,...Ch. 14 - Problem 14.20 Identify A and B, isomers of...Ch. 14 - Problem 14.21 How many signals are present in the ...Ch. 14 - Problem 14.22 What protons in alcohol A give rise...Ch. 14 - How many peaks are observed in the NMR signal for...Ch. 14 -
Problem 14.24 Propose a structure for a compound...Ch. 14 - Problem 14.25 Propose a structure for a compound...Ch. 14 - Problem 14.26. Identify products A and B from the...Ch. 14 - Problem 14.27 How many lines are observed in the ...Ch. 14 - Problem 14.28 Draw all constitutional isomers of...Ch. 14 - Problem 14.29 Esters of chrysanthemic acid are...Ch. 14 - Prob. 14.30PCh. 14 - Problem 14.31 Identify the carbon atoms that give...Ch. 14 - Problem 14.32 A compound of molecular formula ...Ch. 14 - Problem 14.33 Draw the structure of a compound of...Ch. 14 - 14.34 (a) How many NMR signals does each of the...Ch. 14 - 14.35 (a) How many NMR signals does each compound...Ch. 14 - Prob. 14.36PCh. 14 - 14.37 How many NMR signals does each natural...Ch. 14 - Prob. 14.38PCh. 14 - 14.39 What effect does increasing the operating...Ch. 14 - Prob. 14.40PCh. 14 - 14.41 How could you use chemical shift and...Ch. 14 - Prob. 14.42PCh. 14 - 14.43 How can you use NMR spectroscopy to...Ch. 14 - Prob. 14.44PCh. 14 - Prob. 14.45PCh. 14 - Prob. 14.46PCh. 14 - Prob. 14.47PCh. 14 - 14.48 How many NMR signals does each compound...Ch. 14 - 14.49 Rank the highlighted carbon atoms in each...Ch. 14 - 14.50 Identify the carbon atoms that give rise to...Ch. 14 - 14.51 a. How many signals does dimethyl...Ch. 14 - 14.52 Answer the following questions about each of...Ch. 14 - 14.53 Propose a structure consistent with each set...Ch. 14 - 14.54 Identify the structures of isomers A and B...Ch. 14 - 14.55 Reaction of with affords compound W,...Ch. 14 - 14.56 Treatment of with , followed by aqueous
...Ch. 14 - 14.57 Compound C has a molecular ion in its mass...Ch. 14 - 14.58 As we will learn in Chapter 20, reaction of ...Ch. 14 - 14.59 Identify the structures of isomers E and F...Ch. 14 - 14.59 Identify the structures of isomers H and I...Ch. 14 - 14.61 Propose a structure consistent with each set...Ch. 14 - 14.62 Reaction of with , followed by treatment...Ch. 14 - Reaction of aldehyde D with amino alcohol E in the...Ch. 14 - 14.64 Propose a structure consistent with each set...Ch. 14 - 14.65 In the presence of a small amount of acid, a...Ch. 14 - 14.66 Treatment of with affords two products (M...Ch. 14 - 14.67 Compound O has molecular formula and shows...Ch. 14 - 14.68 Compound P has molecular formula . Deduce...Ch. 14 - 14.69 Treatment of with strong base followed by ...Ch. 14 - 14.70 When -bromo--dimethylbutane is treated with...Ch. 14 - 14.71 Propose a structure consistent with each set...Ch. 14 - 14.72 Reaction of unknown A with forms...Ch. 14 - Prob. 14.73PCh. 14 - 14.74 -Annulene shows two signals in its ...Ch. 14 - 14.75 Explain why the spectrum of-methylbutan--ol...Ch. 14 - 14.76 Because has an odd mass number, nuclei...Ch. 14 - 14.77 Cyclohex--enone has two protons on its...
Additional Science Textbook Solutions
Find more solutions based on key concepts
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forwardPlease help me with this the problem is so confusingarrow_forward14 Question (1 point) Disiamylborane adds to a triple bond to give an alkenylborane. Upon oxidation with OH, H2O2, the alkenylborane will form an enol that tautomerizes to an aldehyde. In the first box below, draw the mechanism arrows for the reaction of disiamylborane with the alkyne, and in the last box draw the structure of the aldehyde. 4th attempt Feedback i > 3rd attempt OH, H2O2 i See Periodic Table See Hintarrow_forward
- answer with mechanisms and steps. handwritten please!arrow_forwardHello I need some help with Smartwork. For drawing structure B, I know the correct answer is CH₃B₂, but when I try to type it in, it keeps giving me CH₄BH₃ instead. Do you know how I should write it properly? Should I use a bond or something else?arrow_forwardTrue or false, chemistryarrow_forward
- answer thse questions with mechanisms and steps. handwritten please!arrow_forwardC app.aktiv.com Draw the product of the following reaction sequence. Ignore any inorganic byproducts formed. H O 1. (CH3CH2)2CuLi, THF 2. CH3Br Drawingarrow_forwardDraw the product of the following reaction sequence. Ignore any inorganic byproducts formed. H O 1. (CH3CH2)2CuLi, THF 2. CHзBr Drawingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY