
Chemistry (7th Edition)
7th Edition
ISBN: 9780321943170
Author: John E. McMurry, Robert C. Fay, Jill Kirsten Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 14.5P
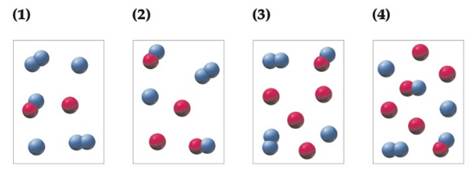
Conceptual PRACTICE 14.5 The following pictures represent mixtures that contain A atoms (red), B atoms (blue), and AB and B2 molecules, which interconvert according to theequation
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Macmillan Learning
Draw the acyl chloride that would give the ketone shown using the Friedel-Crafts acylation reaction.
Select
Draw Templates
More
с H о
Cl
2Q
Erase
AICI₂
Draw the complete mechanism for this reaction:
.OH
مدید
OH
H2SO4
+ H₂O
To save you some time, the starting material has been copied into the first drawing area. However, you will still need to add any other reactants or catalysts that
take part in the reaction.
ན ི..
OH
Add/Remove step
Х
ด
ك
Click and drag to start
drawing a structure.
9:27 AM Tue Mar 4
←
Problem 64 of 15
#63%
Submit
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product
structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s).
Be sure to account for all bond-breaking and bond-making steps.
0:0
0:0
:0:
N.
:0:
:O
:0:
H
H.
:0:
Select to Add Arrows
O
:0:
H
O
:0:
0:0.
S.
H
Select to Add Arrows
S
:0:
:0:
H
H
Chapter 14 Solutions
Chemistry (7th Edition)
Ch. 14 - Prob. 14.1PCh. 14 - APPLY 14.2 Nitrogen dioxide, a pollutant that...Ch. 14 - Prob. 14.3PCh. 14 - APPLY 14.4 Lactic acid, which builds up in muscle...Ch. 14 - Conceptual PRACTICE 14.5 The following pictures...Ch. 14 - Conceptual APPLY 14.6 The equilibrium constant...Ch. 14 - PRACTICE 14.7 In the industrial synthesis of...Ch. 14 - APPLY 14.8 At 25 °C, Kp = 25 for the reaction...Ch. 14 - Prob. 14.9PCh. 14 - APPLY 14.10For the reaction...
Ch. 14 - Prob. 14.11PCh. 14 - APPLY 14.12 Magnesium hydroxide is the active...Ch. 14 - Prob. 14.13PCh. 14 - Prob. 14.14ACh. 14 - Prob. 14.15PCh. 14 - Conceptual APPLY 14.16 The reaction A2 + B2 2...Ch. 14 - PRACTICE 14.17 The H2/CO ratio in mixtures of...Ch. 14 - APPLY 14.18 Calculate the equilibrium...Ch. 14 - PRACTICE 14.19 Calculate the equilibrium...Ch. 14 - APPLY 14.20 Calculate the equilibrium...Ch. 14 - Prob. 14.21PCh. 14 - Prob. 14.22ACh. 14 - Prob. 14.23PCh. 14 - Prob. 14.24ACh. 14 - Prob. 14.25PCh. 14 - Prob. 14.26ACh. 14 - Prob. 14.27PCh. 14 - Prob. 14.28ACh. 14 - Prob. 14.29PCh. 14 - Prob. 14.30ACh. 14 - Prob. 14.31PCh. 14 - Prob. 14.32ACh. 14 - Prob. 14.33PCh. 14 - Prob. 14.34PCh. 14 - Prob. 14.35PCh. 14 - Prob. 14.36PCh. 14 - PROBLEM 14.37 The affinity of hemoglobin (Hb) for...Ch. 14 - Prob. 14.38PCh. 14 - Prob. 14.39CPCh. 14 - The following pictures represent the equilibrium...Ch. 14 - The reaction A2+BA+AB has an equilibrium constant...Ch. 14 - Prob. 14.42CPCh. 14 - Prob. 14.43CPCh. 14 - Prob. 14.44CPCh. 14 - The following pictures represent equilibrium...Ch. 14 - Prob. 14.46CPCh. 14 - Prob. 14.47CPCh. 14 - Prob. 14.48CPCh. 14 - Prob. 14.49CPCh. 14 - Prob. 14.50SPCh. 14 - Identify the true statement about the...Ch. 14 - Prob. 14.52SPCh. 14 - Prob. 14.53SPCh. 14 - For each of the following equilibria, write the...Ch. 14 - Prob. 14.55SPCh. 14 - Prob. 14.56SPCh. 14 - Prob. 14.57SPCh. 14 - For each of the following equilibria, write the...Ch. 14 - Prob. 14.59SPCh. 14 - 14.60 If Kc = 7.5 × 10-9 at 1000 K for the...Ch. 14 - Prob. 14.61SPCh. 14 - Prob. 14.62SPCh. 14 - Prob. 14.63SPCh. 14 - Prob. 14.64SPCh. 14 - Prob. 14.65SPCh. 14 - Prob. 14.66SPCh. 14 - Prob. 14.67SPCh. 14 - Prob. 14.68SPCh. 14 - Prob. 14.69SPCh. 14 - Prob. 14.70SPCh. 14 - Prob. 14.71SPCh. 14 - Prob. 14.72SPCh. 14 - Prob. 14.73SPCh. 14 - Prob. 14.74SPCh. 14 - Prob. 14.75SPCh. 14 - Prob. 14.76SPCh. 14 - Prob. 14.77SPCh. 14 - Prob. 14.78SPCh. 14 - Prob. 14.79SPCh. 14 - Prob. 14.80SPCh. 14 - Prob. 14.81SPCh. 14 - Prob. 14.82SPCh. 14 - Prob. 14.83SPCh. 14 - Prob. 14.84SPCh. 14 - Prob. 14.85SPCh. 14 - Prob. 14.86SPCh. 14 - Prob. 14.87SPCh. 14 - Prob. 14.88SPCh. 14 - Prob. 14.89SPCh. 14 - Prob. 14.90SPCh. 14 - Prob. 14.91SPCh. 14 - Prob. 14.92SPCh. 14 - Prob. 14.93SPCh. 14 - Prob. 14.94SPCh. 14 - Prob. 14.95SPCh. 14 - Prob. 14.96SPCh. 14 - Prob. 14.97SPCh. 14 - Prob. 14.98SPCh. 14 - Prob. 14.99SPCh. 14 - Prob. 14.100SPCh. 14 - Prob. 14.101SPCh. 14 - Prob. 14.102SPCh. 14 - Prob. 14.103SPCh. 14 - Prob. 14.104SPCh. 14 - Consider the endothermic reaction...Ch. 14 - Prob. 14.106SPCh. 14 - Prob. 14.107SPCh. 14 - Prob. 14.108SPCh. 14 - Prob. 14.109SPCh. 14 - Prob. 14.110SPCh. 14 - Prob. 14.111SPCh. 14 - Prob. 14.112SPCh. 14 - Prob. 14.113SPCh. 14 - Prob. 14.114SPCh. 14 - Prob. 14.115SPCh. 14 - Prob. 14.116SPCh. 14 - Prob. 14.117SPCh. 14 - Prob. 14.118SPCh. 14 - Forward and reverse rate constants for the...Ch. 14 - Prob. 14.120CPCh. 14 - Prob. 14.121CPCh. 14 - Prob. 14.122CPCh. 14 - Prob. 14.123CPCh. 14 - Prob. 14.124CPCh. 14 - Prob. 14.125CPCh. 14 - Prob. 14.126CPCh. 14 - Prob. 14.127CPCh. 14 - Prob. 14.128CPCh. 14 - Prob. 14.129CPCh. 14 - Prob. 14.130CPCh. 14 - At 1000 K, Kp, = 2.1 106 and H=107.7kJ for the...Ch. 14 - Prob. 14.132CPCh. 14 - Prob. 14.133CPCh. 14 - Prob. 14.134CPCh. 14 - Prob. 14.135CPCh. 14 - Prob. 14.136CPCh. 14 - Prob. 14.137CPCh. 14 - Prob. 14.138CPCh. 14 - Prob. 14.139CPCh. 14 - Prob. 14.140CPCh. 14 - Prob. 14.141CPCh. 14 - Prob. 14.142CPCh. 14 - Prob. 14.143CPCh. 14 - Prob. 14.144CPCh. 14 - Prob. 14.145CPCh. 14 - Prob. 14.146CPCh. 14 - Prob. 14.147MPCh. 14 - Prob. 14.148MPCh. 14 - Prob. 14.149MPCh. 14 - Prob. 14.150MPCh. 14 - Prob. 14.151MPCh. 14 - Prob. 14.152MPCh. 14 - Prob. 14.153MPCh. 14 - Prob. 14.154MPCh. 14 - Prob. 14.155MPCh. 14 - Prob. 14.156MPCh. 14 - Prob. 14.157MPCh. 14 - Prob. 14.158MPCh. 14 - Prob. 14.159MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Order the following organic reactions by relative rate. That is, select '1' next to the reaction that will have the fastest initial rate, select '2' next to the reaction that will have the next fastest initial rate, and so on. If two reactions will have very similar initial rates, you can select the same number next to both. If a reaction will have zero or nearly zero initial rate, don't select a number and check the box in the table instead. Note: the "Nu" in these reactions means "a generic nucleophile." ملی CI :Nu 2 он 3 H Reaction Relative Rate (Choose one) ▼ Nu :CI: zero or nearly zero Nu :Nu bi (Choose one) zero or nearly zero : Nu لی Nu :H (Choose one) zero or nearly zeroarrow_forward9:12 AM Tue Mar 4 66% Problem 38 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the product formed in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. Br2 FeBrз H (+) Br: H : Br----FeBr3 く a SU 00 nd earrow_forwardUnder aqueous acidic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: ☐ : P Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. CN H₂O H₂O H+ H+ Click and drag to start drawing a structure. Хarrow_forward
- Organic bases have lone pairs of electrons that are capable of accepting protons. Lone pair electrons in a neutral or negatively charged species, or pi electron pairs. Explain the latter case (pi electron pairs).arrow_forwardDescribe the propyl anion.arrow_forwardIndicate the names of these compounds (if they exist). 0: HỌC—NH CH3CH2-CH2arrow_forward
- N Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. NH O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic Garrow_forwardThe conjugate base of alkanes is called alkides. Correct?.arrow_forwardName these organic compounds: structure Br name CH3 CH3 ☐ ☐arrow_forward
- HH H-C H -C-H HH Draw the Skeletal Structures & H Name the molecules HH H H H H-C-C-C-C-C-C-H HHH HHH H H HHHHHHH H-C-C-C-C-C-C-C-C-C-H HHHHH H H H Harrow_forwarddont provide AI solution .... otherwise i will give you dislikearrow_forwardName these organic compounds: structure name CH3 CH3 ☐ F F CH3 ☐ O Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms ofarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY