
Chemistry (7th Edition)
7th Edition
ISBN: 9780321943170
Author: John E. McMurry, Robert C. Fay, Jill Kirsten Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 14.16A
Conceptual APPLY 14.16 The reaction A2 + B2
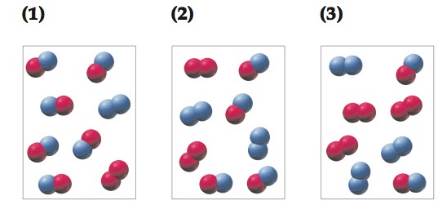
(a) Which reaction mixture is at equilibrium?
(b) For those reaction mixtures that are not at equilibrium, will the net reaction go in the forward or reverse direction to reachequilibrium?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Which of the following is the most acidic transition metal cation?
Group of answer choices
Fe3+
Sc3+
Mn4+
Zn2+
Based on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl?
Group of answer choices
You cannot arrive at any of the other three conclusions
It is a positive value
It is more negative than −0.4 kJ/mol
It equals −0.4 kJ/mol
PLEASE HELP URGENT!
Chapter 14 Solutions
Chemistry (7th Edition)
Ch. 14 - Prob. 14.1PCh. 14 - APPLY 14.2 Nitrogen dioxide, a pollutant that...Ch. 14 - Prob. 14.3PCh. 14 - APPLY 14.4 Lactic acid, which builds up in muscle...Ch. 14 - Conceptual PRACTICE 14.5 The following pictures...Ch. 14 - Conceptual APPLY 14.6 The equilibrium constant...Ch. 14 - PRACTICE 14.7 In the industrial synthesis of...Ch. 14 - APPLY 14.8 At 25 °C, Kp = 25 for the reaction...Ch. 14 - Prob. 14.9PCh. 14 - APPLY 14.10For the reaction...
Ch. 14 - Prob. 14.11PCh. 14 - APPLY 14.12 Magnesium hydroxide is the active...Ch. 14 - Prob. 14.13PCh. 14 - Prob. 14.14ACh. 14 - Prob. 14.15PCh. 14 - Conceptual APPLY 14.16 The reaction A2 + B2 2...Ch. 14 - PRACTICE 14.17 The H2/CO ratio in mixtures of...Ch. 14 - APPLY 14.18 Calculate the equilibrium...Ch. 14 - PRACTICE 14.19 Calculate the equilibrium...Ch. 14 - APPLY 14.20 Calculate the equilibrium...Ch. 14 - Prob. 14.21PCh. 14 - Prob. 14.22ACh. 14 - Prob. 14.23PCh. 14 - Prob. 14.24ACh. 14 - Prob. 14.25PCh. 14 - Prob. 14.26ACh. 14 - Prob. 14.27PCh. 14 - Prob. 14.28ACh. 14 - Prob. 14.29PCh. 14 - Prob. 14.30ACh. 14 - Prob. 14.31PCh. 14 - Prob. 14.32ACh. 14 - Prob. 14.33PCh. 14 - Prob. 14.34PCh. 14 - Prob. 14.35PCh. 14 - Prob. 14.36PCh. 14 - PROBLEM 14.37 The affinity of hemoglobin (Hb) for...Ch. 14 - Prob. 14.38PCh. 14 - Prob. 14.39CPCh. 14 - The following pictures represent the equilibrium...Ch. 14 - The reaction A2+BA+AB has an equilibrium constant...Ch. 14 - Prob. 14.42CPCh. 14 - Prob. 14.43CPCh. 14 - Prob. 14.44CPCh. 14 - The following pictures represent equilibrium...Ch. 14 - Prob. 14.46CPCh. 14 - Prob. 14.47CPCh. 14 - Prob. 14.48CPCh. 14 - Prob. 14.49CPCh. 14 - Prob. 14.50SPCh. 14 - Identify the true statement about the...Ch. 14 - Prob. 14.52SPCh. 14 - Prob. 14.53SPCh. 14 - For each of the following equilibria, write the...Ch. 14 - Prob. 14.55SPCh. 14 - Prob. 14.56SPCh. 14 - Prob. 14.57SPCh. 14 - For each of the following equilibria, write the...Ch. 14 - Prob. 14.59SPCh. 14 - 14.60 If Kc = 7.5 × 10-9 at 1000 K for the...Ch. 14 - Prob. 14.61SPCh. 14 - Prob. 14.62SPCh. 14 - Prob. 14.63SPCh. 14 - Prob. 14.64SPCh. 14 - Prob. 14.65SPCh. 14 - Prob. 14.66SPCh. 14 - Prob. 14.67SPCh. 14 - Prob. 14.68SPCh. 14 - Prob. 14.69SPCh. 14 - Prob. 14.70SPCh. 14 - Prob. 14.71SPCh. 14 - Prob. 14.72SPCh. 14 - Prob. 14.73SPCh. 14 - Prob. 14.74SPCh. 14 - Prob. 14.75SPCh. 14 - Prob. 14.76SPCh. 14 - Prob. 14.77SPCh. 14 - Prob. 14.78SPCh. 14 - Prob. 14.79SPCh. 14 - Prob. 14.80SPCh. 14 - Prob. 14.81SPCh. 14 - Prob. 14.82SPCh. 14 - Prob. 14.83SPCh. 14 - Prob. 14.84SPCh. 14 - Prob. 14.85SPCh. 14 - Prob. 14.86SPCh. 14 - Prob. 14.87SPCh. 14 - Prob. 14.88SPCh. 14 - Prob. 14.89SPCh. 14 - Prob. 14.90SPCh. 14 - Prob. 14.91SPCh. 14 - Prob. 14.92SPCh. 14 - Prob. 14.93SPCh. 14 - Prob. 14.94SPCh. 14 - Prob. 14.95SPCh. 14 - Prob. 14.96SPCh. 14 - Prob. 14.97SPCh. 14 - Prob. 14.98SPCh. 14 - Prob. 14.99SPCh. 14 - Prob. 14.100SPCh. 14 - Prob. 14.101SPCh. 14 - Prob. 14.102SPCh. 14 - Prob. 14.103SPCh. 14 - Prob. 14.104SPCh. 14 - Consider the endothermic reaction...Ch. 14 - Prob. 14.106SPCh. 14 - Prob. 14.107SPCh. 14 - Prob. 14.108SPCh. 14 - Prob. 14.109SPCh. 14 - Prob. 14.110SPCh. 14 - Prob. 14.111SPCh. 14 - Prob. 14.112SPCh. 14 - Prob. 14.113SPCh. 14 - Prob. 14.114SPCh. 14 - Prob. 14.115SPCh. 14 - Prob. 14.116SPCh. 14 - Prob. 14.117SPCh. 14 - Prob. 14.118SPCh. 14 - Forward and reverse rate constants for the...Ch. 14 - Prob. 14.120CPCh. 14 - Prob. 14.121CPCh. 14 - Prob. 14.122CPCh. 14 - Prob. 14.123CPCh. 14 - Prob. 14.124CPCh. 14 - Prob. 14.125CPCh. 14 - Prob. 14.126CPCh. 14 - Prob. 14.127CPCh. 14 - Prob. 14.128CPCh. 14 - Prob. 14.129CPCh. 14 - Prob. 14.130CPCh. 14 - At 1000 K, Kp, = 2.1 106 and H=107.7kJ for the...Ch. 14 - Prob. 14.132CPCh. 14 - Prob. 14.133CPCh. 14 - Prob. 14.134CPCh. 14 - Prob. 14.135CPCh. 14 - Prob. 14.136CPCh. 14 - Prob. 14.137CPCh. 14 - Prob. 14.138CPCh. 14 - Prob. 14.139CPCh. 14 - Prob. 14.140CPCh. 14 - Prob. 14.141CPCh. 14 - Prob. 14.142CPCh. 14 - Prob. 14.143CPCh. 14 - Prob. 14.144CPCh. 14 - Prob. 14.145CPCh. 14 - Prob. 14.146CPCh. 14 - Prob. 14.147MPCh. 14 - Prob. 14.148MPCh. 14 - Prob. 14.149MPCh. 14 - Prob. 14.150MPCh. 14 - Prob. 14.151MPCh. 14 - Prob. 14.152MPCh. 14 - Prob. 14.153MPCh. 14 - Prob. 14.154MPCh. 14 - Prob. 14.155MPCh. 14 - Prob. 14.156MPCh. 14 - Prob. 14.157MPCh. 14 - Prob. 14.158MPCh. 14 - Prob. 14.159MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the skeletal structure corresponding to the following IUPAC name: 7-isopropyl-3-methyldecanearrow_forwardWhich of the following oxyacids is the weakest? Group of answer choices H2SeO3 Si(OH)4 H2SO4 H3PO4arrow_forwardAdd conditions above and below the arrow that turn the reactant below into the product below in a single transformation. + More... If you need to write reagents above and below the arrow that have complex hydrocarbon groups in them, there is a set of standard abbreviations you can use. More... T H,N NC Datarrow_forward
- Indicate the order of basicity of primary, secondary and tertiary amines.arrow_forward> Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. Cl Z- N O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic ○ antiaromatic nonaromaticarrow_forwardPlease help me answer this question. I don't understand how or even if this can happen in a single transformation. Please provide a detailed explanation and a drawing showing how it can happen in a single transformation. Add the necessary reagents and reaction conditions above and below the arrow in this organic reaction. If the products can't be made from the reactant with a single transformation, check the box under the drawing area instead.arrow_forward
- 2) Draw the correct chemical structure (using line-angle drawings / "line structures") from their given IUPAC name: a. (E)-1-chloro-3,4,5-trimethylhex-2-ene b. (Z)-4,5,7-trimethyloct-4-en-2-ol C. (2E,6Z)-4-methylocta-2,6-dienearrow_forwardපිපිම Draw curved arrows to represent the flow of electrons in the reaction on the left Label the reactants on the left as either "Acid" or "Base" (iii) Decide which direction the equilibrium arrows will point in each reaction, based on the given pk, values (a) + H-O H 3-H + (c) H" H + H****H 000 44-00 NH₂ (e) i Дон OH Ө NHarrow_forward3) Label the configuration in each of the following alkenes as E, Z, or N/A (for non-stereogenic centers). 00 E 000 N/A E Br N/A N/A (g) E N/A OH E (b) Oz N/A Br (d) 00 E Z N/A E (f) Oz N/A E (h) Z N/Aarrow_forward
- 6) Fill in the missing Acid, pKa value, or conjugate base in the table below: Acid HCI Approximate pK, -7 Conjugate Base H-C: Hydronium (H₂O') -1.75 H-O-H Carboxylic Acids (RCOOH) Ammonium (NH4) 9.24 Water (H₂O) H-O-H Alcohols (ROH) RO-H Alkynes R--H Amines 25 25 38 HOarrow_forward5) Rank the following sets of compounds in order of decreasing acidity (most acidic to least acidic), and choose the justification(s) for each ranking. (a) OH V SH я вон CH most acidic (lowst pKa) least acidic (highest pKa) Effect(s) Effect(s) Effect(s) inductive effect O inductive effect O inductive effect electronegativity electronegativity O electronegativity resonance polarizability resonance polarizability O resonance O polarizability hybridization Ohybridization O hybridization оarrow_forwardHow negatively charged organic bases are formed.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY