
Bundle: Chemistry for Today: General, Organic, and Biochemistry, Loose-Leaf Version, 9th + LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card
9th Edition
ISBN: 9781337598255
Author: Spencer L. Seager
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.39E
Fructose, present with glucose in honey, reacts with Benedict’s reagent. Circle the structural features that enable fructose to react.
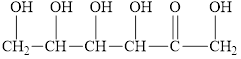
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Which of the following species is a valid resonance structure of A? Use curved arrows to show how A is converted to any valid resonance structure. When a compound is not a valid resonance structurc of A, explain why not.
Provide steps and tips on what to look for to understand how to solve and apply to other problems.
N
IZ
Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under
the table.
Molecule 1
Molecule 2
HN
Molecule 3
Х
HN
www.
Molecule 4
Molecule 5
Molecule 6
none of the above
NH
NH
G
Show work with explanation. don't give Ai generated solution
Chapter 14 Solutions
Bundle: Chemistry for Today: General, Organic, and Biochemistry, Loose-Leaf Version, 9th + LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card
Ch. 14 - Prob. 14.1ECh. 14 - Prob. 14.2ECh. 14 - Identify each of the following compounds as an...Ch. 14 - Identify each of the following compounds as an...Ch. 14 - Prob. 14.5ECh. 14 - Prob. 14.6ECh. 14 - Prob. 14.7ECh. 14 - Prob. 14.8ECh. 14 - Draw structural formulas and give IUPAC names for...Ch. 14 - Draw structural formulas and give IUPAC names for...
Ch. 14 - Each of the following names is wrong. Give the...Ch. 14 - Each of the following names is wrong. Give the...Ch. 14 - Prob. 14.13ECh. 14 - Prob. 14.14ECh. 14 - Prob. 14.15ECh. 14 - Explain why propane boils at 42C, whereas ethanal,...Ch. 14 - Use a dotted line to show hydrogen bonding between...Ch. 14 - Use a dotted line to show hydrogen bonding between...Ch. 14 - Prob. 14.19ECh. 14 - Prob. 14.20ECh. 14 - Prob. 14.21ECh. 14 - Prob. 14.22ECh. 14 - Prob. 14.23ECh. 14 - Prob. 14.24ECh. 14 - Label each of the following as acetals, ketals, or...Ch. 14 - Label each of the following as acetals, ketals, or...Ch. 14 - Label each of the following structures as a cyclic...Ch. 14 - Label each of the following structures as a...Ch. 14 - What two functional groups react to form the...Ch. 14 - Hemiacetals are sometimes referred to as potential...Ch. 14 - Complete the following statements: a. Oxidation of...Ch. 14 - Prob. 14.32ECh. 14 - Prob. 14.33ECh. 14 - Prob. 14.34ECh. 14 - Prob. 14.35ECh. 14 - Not all aldehyde give a positve Bendicts test....Ch. 14 - A stockroom assistant prepares three bottles, each...Ch. 14 - Glucose, the sugar present within the blood, gives...Ch. 14 - Fructose, present with glucose in honey, reacts...Ch. 14 - Prob. 14.40ECh. 14 - Prob. 14.41ECh. 14 - Complete the following equations. If no reaction...Ch. 14 - Complete the following equations. If no reaction...Ch. 14 - Describe the products that result when hydrogen...Ch. 14 - Prob. 14.45ECh. 14 - Draw structural formulas for the products of the...Ch. 14 - The following compounds are cyclic acetals or...Ch. 14 - The following compounds are cyclic acetals or...Ch. 14 - Write equations to show how the following...Ch. 14 - Prob. 14.50ECh. 14 - Identify the most important aldehyde and ketone...Ch. 14 - Using Table 14.3, name an aldehyde or ketone used...Ch. 14 - Prob. 14.53ECh. 14 - CH3COH(O)CH3COOHacetaldehydeaceticacid You need to...Ch. 14 - The addition of water to aldehydes and ketones...Ch. 14 - Prob. 14.56ECh. 14 - Formaldehyde levels above 0.10mg/1000L of ambient...Ch. 14 - In the IUPAC name for the following ketone, it is...Ch. 14 - Why can formaldehyde (CH2O) be prepared in the...Ch. 14 - Other addition reactions of aldehydes occur....Ch. 14 - Prob. 14.61ECh. 14 - Prob. 14.62ECh. 14 - Vanilla flavoring is either extracted from a...Ch. 14 - Prob. 14.64ECh. 14 - The use of acetone in laboratory experiments must...Ch. 14 - Prob. 14.66ECh. 14 - Prob. 14.67ECh. 14 - Which of the following would be classified as a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Follow the curved arrows to draw a second resonance structure for each species. Explain and steps for individual understanding.arrow_forwardDraw all reasonable resonance structures for the following cation. Then draw the resonance hybrid. Provide steps and explanationarrow_forwardHow are the molecules or ions in each pair related? Classify them as resonance structures, isomers, or neither.arrow_forward
- How do I solve this Alkyne synthesis homework problem for my Organic Chemistry II class? I have to provide both the intermediate products and the reagents used.arrow_forwardSubstance X is known to exist at 1 atm in the solid, liquid, or vapor phase, depending on the temperature. Additionally, the values of these other properties of X have been determined: melting point enthalpy of fusion 90. °C 8.00 kJ/mol boiling point 130. °C enthalpy of vaporization 44.00 kJ/mol density 2.80 g/cm³ (solid) 36. J.K mol (solid) 2.50 g/mL (liquid) heat capacity 32. J.Kmol (liquid) 48. J.Kmol (vapor) You may also assume X behaves as an ideal gas in the vapor phase. Ex Suppose a small sample of X at 50 °C is put into an evacuated flask and heated at a constant rate until 15.0 kJ/mol of heat has been added to the sample. Graph the temperature of the sample that would be observed during this experiment. o0o 150- 140 130- 120- 110- 100- G Ar ?arrow_forwardMechanism. Provide the mechanism for the reaction below. You must include all arrows, intermediates, and formal charges. If drawing a Sigma complex, draw all major resonance forms. The ChemDraw template of this document is available on Carmen. Br FeBr3 Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY