
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
10th Edition
ISBN: 9781260028355
Author: Carey
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.7, Problem 10P
The reaction shown gives a single product in
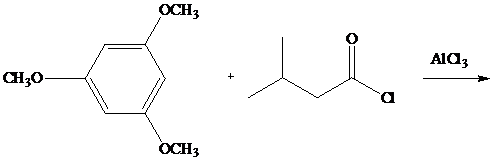
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a structure using wedges and dashes for the following compound:
H-
Et
OH
HO-
H
H-
Me
OH
Which of the following molecules are NOT typical carbohydrates? For the molecules that are
carbohydrates, label them as an aldose or ketose.
HO
Он
ОН ОН
Он
ОН
но
ΤΗ
HO
ОН
HO
eve
Он он
ОН
ОН
ОН
If polyethylene has an average molecular weight of 25,000 g/mol, how many repeat units
are present?
Draw the a-anomer cyclized pyranose Haworth projection of the below hexose. Circle the
anomeric carbons. Number the carbons on the Fischer and Haworth projections. Assign R
and S for each chiral center.
HO
CHO
-H
HO
-H
H-
-OH
H
-OH
CH₂OH
Draw the ẞ-anomer cyclized furanose Haworth projection for the below hexose. Circle the
anomeric carbons. Number the carbons on the Fischer and Haworth projections.
HO
CHO
-H
H
-OH
HO
-H
H
-OH
CH₂OH
Chapter 13 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
Ch. 13.2 - Based on Hammonds postulate which holds that the...Ch. 13.3 - Prob. 2PCh. 13.3 - Using : O =N+= O : as the electrophile, write a...Ch. 13.4 - Prob. 4PCh. 13.5 - Prob. 5PCh. 13.6 - Prob. 6PCh. 13.6 - Write a reasonable mechanism for the formation of...Ch. 13.6 - tert-Butylbenzene can be prepared by alkylation of...Ch. 13.6 - Prob. 9PCh. 13.7 - The reaction shown gives a single product in 88...
Ch. 13.7 - Prob. 11PCh. 13.8 - Using benzene and any necessary organic or...Ch. 13.10 - Prob. 13PCh. 13.11 - Prob. 14PCh. 13.12 - Prob. 15PCh. 13.12 - Prob. 16PCh. 13.13 - Prob. 17PCh. 13.13 - Prob. 18PCh. 13.14 - Reaction of chlorobenzene with p-chlorobenzyl...Ch. 13.15 - Prob. 20PCh. 13.15 - Prob. 21PCh. 13.15 - Prob. 22PCh. 13.16 - Prob. 23PCh. 13.16 - Prob. 24PCh. 13.17 - Prob. 25PCh. 13.18 - Prob. 26PCh. 13.19 - Write the structure of the expected product from...Ch. 13.20 - Prob. 28PCh. 13.20 - Prob. 29PCh. 13.21 - Prob. 30PCh. 13.21 - Offer an explanation for the observation that...Ch. 13.21 - Prob. 32PCh. 13 - Write the structure of the organic product in each...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Prob. 36PCh. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Treatment of the alcohol shown with sulphuric acid...Ch. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - Arrange the following five compounds in order of...Ch. 13 - Prob. 45PCh. 13 - Prob. 46PCh. 13 - Prob. 47PCh. 13 - Give reagents suitable for carrying out each of...Ch. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Which is the best synthesis of the compound shown?Ch. 13 - What combination of acyl chloride or acid...Ch. 13 - A standard synthetic sequence for building a...Ch. 13 - Prob. 54PCh. 13 - Prob. 55PCh. 13 - Prob. 56PCh. 13 - Prob. 57PCh. 13 - Prob. 58PCh. 13 - Prob. 59PCh. 13 - Prob. 60DSPCh. 13 - Prob. 61DSPCh. 13 - Prob. 62DSPCh. 13 - Prob. 63DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the below disaccharide. Circle any hemiacetals. Identify the numbering of glycosidic linkage, and identify it as a or ẞ. OH HO HO OH HO HO HO OHarrow_forwardWhat are the monomers used to make the following polymers? F. а. b. с. d. Вецер хочому なarrow_forward1. Propose a reasonable mechanism for the following transformation. I'm looking for curved mechanistic arrows and appropriate formal charges on intermediates. OMe MeO OMe Me2N NMe2 OTBS OH xylenes OMe 'OTBSarrow_forward
- What is the polymer made from the following monomers? What type of polymerization is used for each? а. ОН H2N но b. ن -NH2 d. H₂N NH2 довarrow_forwardCondensation polymers are produced when monomers containing two different functional groups link together with the loss of a small molecule such as H2O. The difunctional monomer H2N(CH2)6COOH forms a condensation polymer. Draw the carbon-skeleton structure of the dimer that forms from this monomer.arrow_forwardWhat is the structure of the monomer?arrow_forward
- → BINDERIYA GANBO... BINDERIYA GANBO. AP Biology Notes Gamino acid chart - G... 36:22 司 10 ☐ Mark for Review Q 1 Hide 80 8 2 =HA O=A¯ = H₂O Acid HIO HBrO HCIO Question 10 of 35 ^ Σ DELL □ 3 % Λ & 6 7 * ∞ 8 do 5 $ 4 # m 3 ° ( 9 Highlights & Notes AXC Sign out Carrow_forwardWhich representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forward
- Please answer 1, 2 and 3 on the endarrow_forwardIn the box below, specify which of the given compounds are very soluble in polar aprotic solvents. You may select more than one compound. Choose one or more: NaCl NH4Cl CH3CH2CH2CH2CH2CN CH3CH2OH hexan-2-one NaOH CH3SCH3arrow_forwardOn the following structure, select all of the atoms that could ACCEPT a hydrogen bond. Ignore possible complications of aromaticity. When selecting be sure to click on the center of the atom.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License