
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
3rd Edition
ISBN: 9780133858501
Author: Bruice
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13.5, Problem 13P
(a)
Interpretation Introduction
Interpretation:
Aldehyde or ketone that is obtained when the given compound is heated in basic aqueous solution to be identified.
Concept introduction:
Aldol reaction is an addition reaction of
Mechanism for the aldol addition is given as,
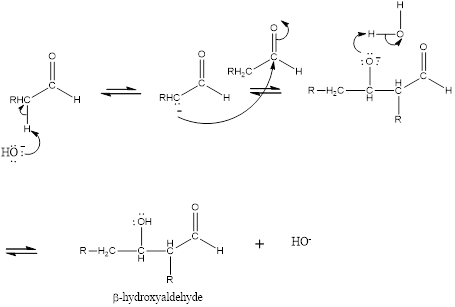
(b)
Interpretation Introduction
Interpretation:
Aldehyde or ketone that is obtained when the given compound is heated in basic aqueous solution to be identified.
Concept introduction:
Aldol reaction is an addition reaction of aldehydes and ketones.
Mechanism for the aldol addition:

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. What is the functional group of an alcohol and a phenol?
2. Why are some alcohols soluble in water?
3. Classify each of the following alcohols as primary, secondary or tertiary.
a. 3-pentanol
b. 2-methyl-2-butanol
c. 1-propanol
I need help with B2 using the information in B1. This is for my lab notebook, and I got confused on number 2. Please help.
4.
Aluminum has a face-centered cubic structure. The unit cell length is 4.05Å. Calculate
the radius of Al atom in the metal.
(5 points).
Chapter 13 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
Ch. 13.1 - Identify the most acidic hydrogen in each...Ch. 13.1 - Prob. 2PCh. 13.1 - Prob. 3PCh. 13.1 - Prob. 4PCh. 13.1 - Explain why HO cannot remove a proton from the...Ch. 13.2 - Prob. 6PCh. 13.2 - Prob. 7PCh. 13.3 - Prob. 8PCh. 13.3 - Prob. 9PCh. 13.3 - Prob. 10P
Ch. 13.4 - Prob. 11PCh. 13.5 - Prob. 12PCh. 13.5 - Prob. 13PCh. 13.6 - Prob. 14PCh. 13.7 - Prob. 16PCh. 13.8 - Prob. 17PCh. 13.8 - Prob. 18PCh. 13.8 - Prob. 19PCh. 13.9 - Prob. 20PCh. 13.10 - Propose a mechanism for the formation of...Ch. 13.10 - Prob. 22PCh. 13.10 - a. If the biosynthesis of palmitic acid were...Ch. 13 - Draw the enol tautomers for each of the following...Ch. 13 - Number the following compounds in order from...Ch. 13 - Prob. 26PCh. 13 - Explain why the pKa of a hydrogen bonded to the...Ch. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - Prob. 31PCh. 13 - Prob. 32PCh. 13 - Prob. 33PCh. 13 - Using cyclopentanone as the reactant, show the...Ch. 13 - Prob. 35PCh. 13 - Prob. 36PCh. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - Prob. 44PCh. 13 - Describe how the following compounds can be...Ch. 13 - Prob. 46PCh. 13 - Which would require a higher temperature:...Ch. 13 - Prob. 48PCh. 13 - Propose a mechanism for the following reaction:Ch. 13 - Show how the following compounds could be...Ch. 13 - Prob. 51PCh. 13 - Prob. 52P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- HF and HNO2 are both considered weak acids. Given the following K values for their dissociationequations, which is the weaker of these two weak acids?HF (aq) ⇌ H+(aq) + F –(aq) K=6.6 x10-4 HNO2 (aq) ⇌ H+(aq) + NO2–(aq) K=7.2 X 10-4arrow_forwardThe equilibrium constant for this reaction is 5.88 x 104. If concentration of the lead ion is 5.24 M, whatis the concentration of the chloride ion?Pb2+(aq) + 2 Cl- (aq) ⇌ PbCl2(s)arrow_forwardc. 1-propanoi 4. If you add chromate, an oxidizing agent, to each of the following, would a green Cr3+ solution be formed? a. 3-pentanol b. 2-methyl-2-butanol c. 1-propanol 5. If an alcohol solution has a pH of 5, would it be a primary alcohol, a secondary alcohol, a tertiary alcohol, or a phenol?arrow_forward
- Given the reaction: A(aq) + B(aq) ⇌ 2C(aq) + D(aq). 2.00 moles of each reactant were dissolved into 1.00 literof water. The reaction reached equilibrium, and at equilibrium the concentration of A was 1.60 M.A) Calculate the equilibrium concentrations for each substance. B) Write the equilibrium constant expression. C) Calculate the value for the equilibrium constant, Keq.arrow_forward1) Draw the structures of D-lysine and L-lysine and assign R/S configuration (showing your workings). 2) Draw the predominant ionisation forms of the free amino acid lysine, at pH 1.0, 8.0, and 11.0. pKa values: 2.2 (-COOH), 9.0 (α-NH3+), 10.5 (side-chain). 3) Calculate (showing your workings) the % of the different ionized species that are present in a 1.00 M solution of L-proline at pH = 10.0. pKa values: 1.95 (- COOH), 10.64 (α-NH3*). 4) a) Draw the tripeptide Tyr-Pro-Lys once with a trans peptide bond between Tyr and Pro and once with a cis peptide bond between Tyr and Pro. b) The electrospray ionization mass spectrum (ESI-MS) of the tripeptide you designed in part (a) shows peaks indicative of mono-protonation and di- protonation of the tripeptide. At what values of m/z would these peaks be expected (no fragmentation)? Briefly explain your answer (showing your workings). 5) How could the sequence of Ala-Met-Thr be distinguished from that of Thr-Ala- Met by tandem ESI-MS-MS?…arrow_forwardLABORATORY REPORT FORM Part I. Determination of the Formula of a Known Hydrate 1. Mass of empty evaporating dish 3. Mass of hydrate Using subtraction or mass by difference, find the mass of the hydrate 76.96 -75.40 75.40g 76.968 1.568 01.56 76.90 g 2. Mass of evaporating dish + hydrate 4. Mass of evaporating dish + hydrate (after heating) First 76.98 g Third 76.66g Second Fourth (if necessary) 76.60g 5. Mass of anhydrate 6. Mass of water lost by the hydrate 7. Percent of water of hydration (Show Calculations) 8. Moles of water (Show Calculations) mol mass of water = MM of water (g/m) 9. Moles of anhydrate (Show Calculations) 10. Ratio of moles of water to moles of anhydrate 11 F(Show Calculations) 11. Formula of hydrate - Mass of water (g) x 100 % water hydration g g % Mass of hydrate (9) x IC % = (Mass of hydrate- mass of an) mass of hydrate (g) % = (1.569- × 100= mol 1.569 mol Mol Mass of anhydrate/MM of anhydrate 12. What was the color of the hydrate? blue What was the color of the…arrow_forward
- compared t-critical with t-calculated and 95% confidence interval to answer this questionarrow_forwardComparing two means. Horvat and co-workers used atomic absorption spectroscopy to determine the concentration of Hg in coal fly ash. Of particular interest to the authors was developing an appropriate procedure for digesting samples and releasing the Hg for analysis. As part of their study they tested several reagents for digesting samples. Their results using HNO3 and using a 1+3 mixture of HNO3 and HCl are shown here. All concentrations are given as ppb Hg sample. HNO3: 161, 165, 160, 167, 166 1+3 HNO3–HCl: 159, 145, 140, 147, 143, 156 Determine whether there is a significant difference between these methods at the 95% confidence interval.arrow_forwardComparison of experimental data to “known” value. Monna and co-workers used radioactive isotopes to date sediments from lakes and estuaries.21 To verify this method they analyzed a 208Po standard known to have an activity of 77.5 decays/min, obtaining the following results. 77.09, 75.37, 72.42, 76.84, 77.84, 76.69, 78.03, 74.96, 77.54, 76.09, 81.12, 75.75 Do the results differ from the expected results at the 95% confidence interval?arrow_forward
- Explain the difference between the propagated uncertainty and the standard deviation. Which number would you use to describe the uncertainty in the measurement? if the standard deviation is 0.01 and the propagated uncertainty is 0.03arrow_forwardPropagation of uncertainty. Find the absolute and percent relative uncertainty assuming the ±-values are random error. 7.65±0.04 + 5.28±0.02 – 1.12±0.01 85.6±0.9 × 50.2±0.7 ÷ 13.8±0.5 [4.88±0.07 + 3.22±0.05] / 1.53±0.02arrow_forwardExplain the difference between the propagated uncertainty and the standard deviation. Which number would you use to describe the uncertainty in the measurement?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY