
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.5, Problem 1.1ACP
The blue line on the diagram illustrates the effect of using fractional distillation to separate a mixture of hexane (C6H14) and heptane (C7H16). If one starts a fractional distillation with a 0.20 mole fraction of hexane, what is the approximate mole fraction of hexane in the vapor phase after two evaporation condensation cycles?
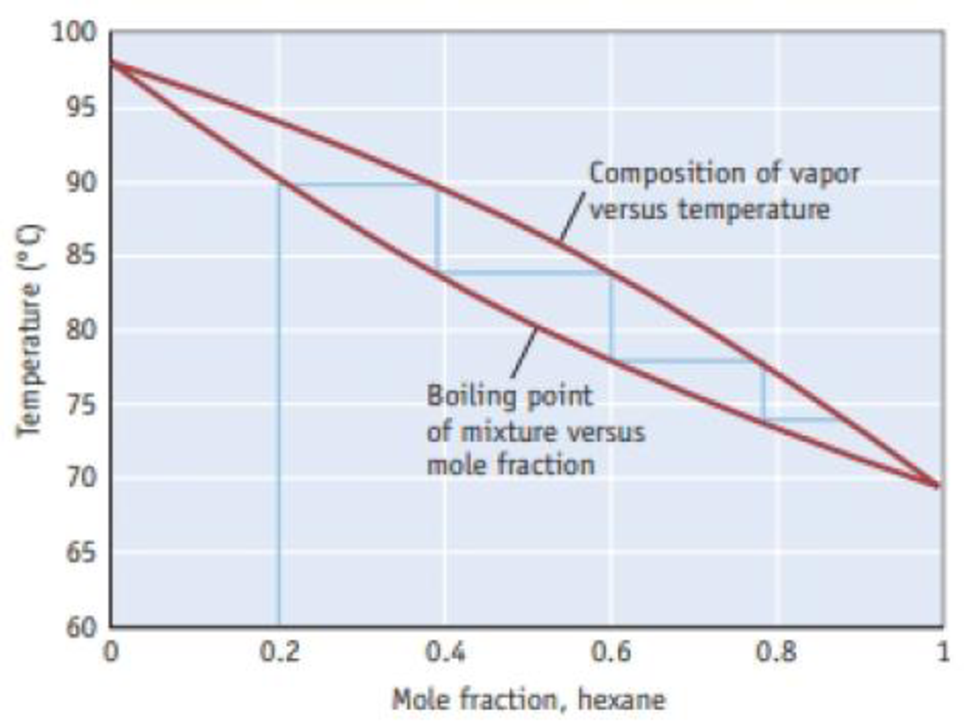
Figure B A temperature–composition diagram for hexane (C6H14)-heptane (C7H16) mixtures.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes
*The values are all provided in the photo attached*
pressure (atm)
3
The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the
temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes.
0
0
200
temperature (K)
400
а
er your payment details | bar xb Home | bartleby
x +
aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1
O States of Matter
Sketching a described thermodynamic change on a phase diagram
0/5
The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the
temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes.
pressure (atm)
1
3-
0-
0
200
Explanation
Check
temperature (K)
400
X
Q Search
L
G
2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Ce
Chapter 13 Solutions
Chemistry & Chemical Reactivity
Ch. 13.1 - (a) If you dissolve 10.0 g (about one heaping...Ch. 13.2 - Use the data in Table 13.1 to calculate the...Ch. 13.3 - Prob. 13.3CYUCh. 13.4 - Assume you dissolve 10.0 g of sucrose (C12H22O11)...Ch. 13.4 - What quantity of ethylene glycol, HOCH2CH2OH, must...Ch. 13.4 - In the northern United States, summer cottages are...Ch. 13.4 - Bradykinin is a small peptide (9 amino acids; 1060...Ch. 13.4 - An aluminum-containing compound has the empirical...Ch. 13.4 - A 1.40-g sample of polyethylene, a common plastic,...Ch. 13.4 - Calculate the freezing point of 525 g of water...
Ch. 13.5 - The blue line on the diagram illustrates the...Ch. 13.5 - How many theoretical plates are required to...Ch. 13.5 - Prob. 1.3ACPCh. 13.5 - The vapor pressure of pure heptane is 361.5 mm Hg...Ch. 13.5 - If the headspace of a soda is 25 mL and the...Ch. 13.5 - Prob. 2.2ACPCh. 13.5 - Prob. 2.3ACPCh. 13.5 - Prob. 2.4ACPCh. 13.5 - Prob. 3.1ACPCh. 13.5 - Prob. 3.2ACPCh. 13.5 - Prob. 3.3ACPCh. 13 - You dissolve 2.56 g of succinic acid, C2H4(CO2H)2,...Ch. 13 - You dissolve 45.0 g of camphor, C10H16O, in 425 mL...Ch. 13 - Prob. 3PSCh. 13 - Prob. 4PSCh. 13 - Prob. 5PSCh. 13 - Prob. 6PSCh. 13 - Prob. 7PSCh. 13 - Prob. 8PSCh. 13 - Hydrochloric acid is sold as a concentrated...Ch. 13 - Concentrated sulfuric acid has a density of 1.84...Ch. 13 - The average lithium ion concentration in seawater...Ch. 13 - Silver ion has an average concentration of 28 ppb...Ch. 13 - Which pairs of liquids will be miscible? (a) H2O...Ch. 13 - Acetone, CH3COCH3, is quite soluble in water....Ch. 13 - Prob. 15PSCh. 13 - Use the following data to calculate the enthalpy...Ch. 13 - You make a saturated solution of NaCl at 25 C. No...Ch. 13 - Some lithium chloride, LiCl, is dissolved in 100...Ch. 13 - Prob. 19PSCh. 13 - The Henrys law constant for O2 in water at 25 is...Ch. 13 - An unopened soda can has an aqueous CO2...Ch. 13 - Hydrogen gas has a Henrys law constant of 7.8 104...Ch. 13 - A sealed flask contains water and oxygen gas at 25...Ch. 13 - Butane, C4H10, has been suggested as the...Ch. 13 - A 35.0-g sample of ethylene glycol, HOCH2CH2OH, is...Ch. 13 - Urea, (NH2)2CO, which is widely used in...Ch. 13 - Pure ethylene glycol, HOCH2CH2OH, is added 2.00 kg...Ch. 13 - Pure iodine (105 g) is dissolved in 325 g of CCl4...Ch. 13 - Prob. 29PSCh. 13 - What is the boiling point of a solution composed...Ch. 13 - Prob. 31PSCh. 13 - Prob. 32PSCh. 13 - Prob. 33PSCh. 13 - Some ethylene glycol, HOCH2CH2OH, is added to your...Ch. 13 - You dissolve 15.0 g of sucrose, C12H22O11, in a...Ch. 13 - A typical bottle of wine consists of an 11%...Ch. 13 - Prob. 37PSCh. 13 - Estimate the osmotic pressure of human blood at 37...Ch. 13 - An aqueous solution containing 1.00 g of bovine...Ch. 13 - Calculate the osmotic pressure of a 0.0120 M...Ch. 13 - You add 0.255 g of an orange, crystalline compound...Ch. 13 - Butylated hydroxyanisole (BHA) is used in...Ch. 13 - Benzyl acetate is one of the active components of...Ch. 13 - Anthracene, a hydrocarbon obtained from coal, has...Ch. 13 - An aqueous solution contains 0.180 g of an...Ch. 13 - Aluminon, an organic compound, is used as a...Ch. 13 - Prob. 47PSCh. 13 - To make homemade ice cream, you cool the milk and...Ch. 13 - List the following aqueous solutions in order of...Ch. 13 - Arrange the following aqueous solutions in order...Ch. 13 - When solutions of BaCl2 and Na2SO4 are mixed, the...Ch. 13 - The dispersed phase of a certain colloidal...Ch. 13 - Phenylcarbinol is used in nasal sprays as a...Ch. 13 - (a) Which aqueous solution is expected to have the...Ch. 13 - Arrange the following aqueous solutions in order...Ch. 13 - Prob. 56GQCh. 13 - Dimethylglyoxime [DMG, (CH3CNOH)2] is used as a...Ch. 13 - A 10.7 m solution of NaOH has a density of 1.33...Ch. 13 - Concentrated aqueous ammonia has a molarity of...Ch. 13 - Prob. 60GQCh. 13 - If you want a solution that is 0.100 m in ions,...Ch. 13 - Consider the following aqueous solutions: (i) 0.20...Ch. 13 - (a) Which solution is expected to have the higher...Ch. 13 - The solubility of NaCl in water at 100 C is 39.1...Ch. 13 - Instead of using NaCl to melt the ice on your...Ch. 13 - The smell of ripe raspberries is due to...Ch. 13 - Hexachlorophene has been used in germicidal soap....Ch. 13 - The solubility of ammonium formate, NH4CHO2, in...Ch. 13 - How much N2 can dissolve in water at 25 C if the...Ch. 13 - Cigars are best stored in a humidor at 18 C and...Ch. 13 - An aqueous solution containing 10.0 g of starch...Ch. 13 - Prob. 72GQCh. 13 - Calculate the enthalpies of solution for Li2SO4...Ch. 13 - Water at 25 C has a density of 0.997 g/cm3....Ch. 13 - If a volatile solute is added to a volatile...Ch. 13 - A solution is made by adding 50.0 mL of ethanol...Ch. 13 - A 2.0% (by mass) aqueous solution of novocainium...Ch. 13 - A solution is 4.00% (by mass) maltose and 96.00%...Ch. 13 - The following table lists the concentrations of...Ch. 13 - A tree is 10.0 m tall. (a) What must be the total...Ch. 13 - Prob. 81GQCh. 13 - A compound is known to be a potassium halide, KX....Ch. 13 - Prob. 85GQCh. 13 - If one is very careful, it is possible to float a...Ch. 13 - A solution of benzoic acid in benzene has a...Ch. 13 - You dissolve 5.0 mg of iodine, I2, in 25 mL of...Ch. 13 - Prob. 89ILCh. 13 - In a police forensics lab, you examine a package...Ch. 13 - An organic compound contains carbon (71.17%),...Ch. 13 - Prob. 92ILCh. 13 - When sails of Mg2+, Ca2+, and Be2+ are placed in...Ch. 13 - Explain why a cucumber shrivels up when it is...Ch. 13 - Prob. 95SCQCh. 13 - A 100.-gram sample of sodium chloride (NaCl) is...Ch. 13 - Prob. 97SCQCh. 13 - Prob. 98SCQCh. 13 - Starch contains CC, CH, CO, and OH bonds....Ch. 13 - Prob. 100SCQCh. 13 - You have two aqueous solutions separated by a...Ch. 13 - Prob. 102SCQCh. 13 - Sodium chloride (NaCl) is commonly used to melt...Ch. 13 - Prob. 105SCQCh. 13 - Prob. 106SCQCh. 13 - Prob. 107SCQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
Some people consider Pasteur or Koch to be the Father of Microbiology, rather than Leeuwenhoek. Why might they ...
Microbiology with Diseases by Body System (5th Edition)
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
Match the people in column A to their contribution toward the advancement of microbiology, in column B. Column ...
Microbiology: An Introduction
Some people compare DNA to a blueprint stored in the office of a construction company. Explain how this analogy...
Biology: Concepts and Investigations
11. In the early 1800s, French naturalist Jean Baptiste Lamarck suggested that the best explanation for the rel...
Campbell Biology: Concepts & Connections (9th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5.arrow_forward6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward
- 5.arrow_forward9arrow_forwardalekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward
- 0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forwardx + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forwardS: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward
- 151.2 254.8 85.9 199.6 241.4 87.6 242.5 186.4 155.8 257.1 242.9 253.3 256.0 216.6 108.7 239.0 149.7 236.4 152.1 222.7 148.7 278.2 268.7 234.4 262.7 283.2 143.6 QUESTION: Using this group of data on salt reduced tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardResults Search Results Best Free Coursehero Unloc xb Success Confirmation of Q x O Google Pas alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J O States of Matter Using a phase diagram to find a phase transition temperature or pressure Gabr 3/5 he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to nd your answer. pressure (atm) 24- 12 solid liquid gas 200 400 temperature (K) 600 ote: your answer must be within 0.15 atm of the exact answer to be graded correct. atm Thanation Check © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Q Search L³ ملةarrow_forward301.7 348.9 193.7 308.6 339.5 160.6 337.7 464.7 223.5 370.5 326.6 327.5 336.1 317.9 203.8 329.8 221.9 331.7 211.7 309.6 223.4 353.7 334.6 305.6 340.0 304.3 244.7 QUESTION: Using this group of data on regular tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY