
Concept explainers
An insulated tank that contains 1 kg of O2 at 15°C and 300 kPa is connected to a 2-m3 uninsulated tank that contains N2 at 50°C and 500 kPa. The valve connecting the two tanks is opened, and the two gases form a homogeneous mixture at 25°C. Determine (a) the final pressure in the tank, (b) the heat transfer, and (c) the entropy generated during this process. Assume T0 = 25°C.
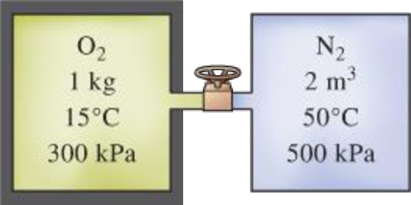
FIGURE P13–56
(a)
The pressure of the mixture.
Answer to Problem 57P
The pressure of the mixture is
Explanation of Solution
Refer to Table A-2, obtain the constant-volume specific heats of the gases at room temperature.
Write the equation to calculate the volume of the oxygen tank.
Here, mass of oxygen tank is
Calculate the mass of nitrogen gas.
Here, initial temperature and pressure of nitrogen gas is
Calculate the total volume.
Calculate the mole numbers of
Here, molar mass of
Calculate the mole number of the mixture.
Calculate the pressure of the mixture.
Here, universal gas constant of the mixture is
Conclusion:
Refer to Table A-1, obtain the gas constants of
Substitute 1 kg for
Substitute
Substitute
Refer to Table A-1, obtain the molar mass of
Substitute 1 kg for
Substitute 10.43 kg for
Substitute
Substitute
Thus, the pressure of the mixture is
(b)
The heat transfer.
Answer to Problem 57P
The heat transfer is
Explanation of Solution
Write the equation of energy balance for a closed system.
Here, heat output is
Conclusion:
Substitute 1 kg for
Thus, the heat transfer is
(c)
The entropy generation.
Answer to Problem 57P
The entropy generation is
Explanation of Solution
Write the equation of entropy balance.
Here, entropy at inlet and exit is
Calculate the mole fraction of
Calculate the value of
Here, the partial pressure of mixture at state 2 is
Calculate the value of
Here, the partial pressure of mixture at state 2 is
Conclusion:
Substitute
Substitute
Refer to Table A-2, obtain the constant-pressure specific heats of the gases at room temperature.
Substitute 0.077 for
Substitute 0.923 for
Substitute
Thus, the entropy generation is
Want to see more full solutions like this?
Chapter 13 Solutions
THERMODYNAMICS(SI UNITS,INTL.ED)EBOOK>I
- Determine the entropy change for the mixing process, in kJ/K.arrow_forwardDetermine the entropy change of 1.5 moles of ammonia that is heated from 180°C to 750°C. The system operates at an atmospheric pressure on a steady flow process.arrow_forwardA certain polyatomic gas stored at a 180-L rigid tank and 11 atm is heated at constant volume from 35 ⁰C to 75 ⁰C. Determine the change in entropy (in J/K).arrow_forward
- Water is contained in a closed rigid tank at an initial pressure of 1200 kPa. Heat transfer occurs until the pressure increases to 7 MPa and the tank found to be containing 1,78 kg saturated liquid, and the mass of the saturated vapor is 0.22 kg. Determine the initial temperature, entropy and enthalpy of the water.arrow_forwardTwo tanks (Tank A and Tank B) are separated by a partition. Initially Tank A contains 2.6 kg steam at 5 MPa and 400°C while Tank B contains 4.8 kg saturated liquid-vapor mixture at 140°C with a quality of 47%. Now the partition is removed and the two sides are allowed to mix until the mechanical and thermal equilibrium are established. If the pressure at the final state is 275 kPa, determine (a) the temperature and quality of the steam (if mixture) at the final state and (b) the amount of heat lost from the tanks. Aarrow_forwardSaturated water at 36.6°C passes through a throttling valve and becomes a mixture of water and steam at 5.4°C. Calculate the specific volume and specific enthalpy of the mixture. Show detailed steps of how the properties are obtained by using only the Saturated Water and Steam table. Consider that a throttling process is a typical irreversible process. The specific enthalpies of the working fluid before and after a throttling valve are the same. The steam table has been provided in the imagearrow_forward
- Nitogen is cooled at a constant pressure of 1200 Kpa from an initial specific volume of 0.76 m3/Kg. If the mass of Nitrogen undergoing the process is 2.20 Kgs. Determine: a.The work, in KJ b.The change in Entropy, in KJ/K c.The change in enthalpy and internal energy, in KJarrow_forward4. A 0.5 m³ rigid tank contains refrigerant-134a initially at 200 kPa and 40 percent quality. Heat is now transferred to the refrigerant until the pressure reaches 800 kPa. Determine (a) the mass of the refrigerant in the tank and (b) the amount of heat transferred. Also, show the process on a P-v diagram with respect to saturation lines. [12.3 kg, 2956.2Kj]arrow_forwardHelp please on allarrow_forward
- An insulated rigid tank is divided into two equal parts by a partition. Initially, one part contains 14.5 kmol of an ideal gas at 330 kPa and 50°C, and the other side is evacuated. The partition is now removed, and the gas fills the entire tank. Determine the total entropy change during this process. The value of the universal gas constant Ru is 8.314 kJ/kmol-K. The total entropy change during this process is kJ/K.arrow_forwardA mixture of hydrocarbon gases is composed of 60 percent methane, 25 percent propane, and 15 percent butane by weight. This mixture is compressed from 100 kPa and 20°C to 1400 kPa in a reversible, isothermal, steady-flow compressor. Calculate the work and heat transfer for this compression per unit mass of the mixture. The universal gas constant is R₁ = 8.314 kPa-m³/kmol-K. Use the table containing the molar mass, gas constant, and critical-point properties. P₂ 60% CH4 25% C₂H₂ 15% C₂H10 (by mass) 100 kPa 20°C W The work input for this compression per unit mass of the mixture is The heat transfer for this compression per unit mass of the mixture is kJ/kg. kJ/kg.arrow_forwardArgon gas is contained in a cylinder fitted with a frictionless piston. Initially, the cylinder contains 200 L of Argon at 140 kPa and 10o C. The gas is then compressed in a polytropic process according to the relationship Pvn = C until the final pressure and temperature are 700 kPa and 180o C respectively. For Argon; R = 0.2081 kJ/kg.K and cv = 0.3122 kJ/kg.K. i) Sketch the system and the details of the process. ii) Show the process on a P-v diagram iii) Determine the polytropic exponent, n iv) Calculate the work involved during the process [kJ] v) Calculate the heat transfer during this process [kJ]arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





