
Thermodynamics: An Engineering Approach ( 9th International Edition ) ISBN:9781260092684
9th Edition
ISBN: 9781260048667
Author: Yunus A. Cengel Dr.; Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 13.3, Problem 33P
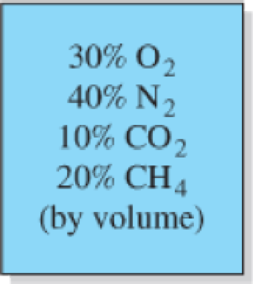
The volumetric analysis of a mixture of gases is 30 percent oxygen, 40 percent nitrogen, 10 percent carbon dioxide, and 20 percent methane. Calculate the apparent specific heats and molecular weight of this mixture of gases.
FIGURE P13–33
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Q11. Determine the magnitude of the reaction force at C.
1.5 m
a)
4 KN
D
b)
6.5 kN
c)
8 kN
d)
e)
11.3 KN
20 kN
-1.5 m-
C
4 kN
-1.5 m
B
Mechanical engineering, No
Chatgpt.
please help with this practice problem(not a graded assignment, this is a practice exam), and please explain how to use sohcahtoa
Solve this problem and show all of the work
Chapter 13 Solutions
Thermodynamics: An Engineering Approach ( 9th International Edition ) ISBN:9781260092684
Ch. 13.3 - What are mass and mole fractions?Ch. 13.3 - Consider a mixture of several gases of identical...Ch. 13.3 - The sum of the mole fractions for an ideal-gas...Ch. 13.3 - Somebody claims that the mass and mole fractions...Ch. 13.3 - Consider a mixture of two gases. Can the apparent...Ch. 13.3 - What is the apparent molar mass for a gas mixture?...Ch. 13.3 - Prob. 7PCh. 13.3 - The composition of moist air is given on a molar...Ch. 13.3 - Prob. 9PCh. 13.3 - Prob. 10P
Ch. 13.3 - A gas mixture consists of 20 percent O2, 30...Ch. 13.3 - Prob. 12PCh. 13.3 - Prob. 13PCh. 13.3 - Consider a mixture of two gases A and B. Show that...Ch. 13.3 - Is a mixture of ideal gases also an ideal gas?...Ch. 13.3 - Express Daltons law of additive pressures. Does...Ch. 13.3 - Express Amagats law of additive volumes. Does this...Ch. 13.3 - Prob. 18PCh. 13.3 - How is the P-v-T behavior of a component in an...Ch. 13.3 - Prob. 20PCh. 13.3 - Prob. 21PCh. 13.3 - Prob. 22PCh. 13.3 - Consider a rigid tank that contains a mixture of...Ch. 13.3 - Prob. 24PCh. 13.3 - Is this statement correct? The temperature of an...Ch. 13.3 - Is this statement correct? The volume of an...Ch. 13.3 - Is this statement correct? The pressure of an...Ch. 13.3 - A gas mixture at 300 K and 200 kPa consists of 1...Ch. 13.3 - Prob. 29PCh. 13.3 - Separation units often use membranes, absorbers,...Ch. 13.3 - Prob. 31PCh. 13.3 - The mass fractions of a mixture of gases are 15...Ch. 13.3 - The volumetric analysis of a mixture of gases is...Ch. 13.3 - An engineer has proposed mixing extra oxygen with...Ch. 13.3 - A rigid tank contains 0.5 kmol of Ar and 2 kmol of...Ch. 13.3 - A mixture of gases consists of 0.9 kg of oxygen,...Ch. 13.3 - Prob. 37PCh. 13.3 - One pound-mass of a gas whose density is 0.001...Ch. 13.3 - A 30 percent (by mass) ethane and 70 percent...Ch. 13.3 - Prob. 40PCh. 13.3 - Prob. 41PCh. 13.3 - A rigid tank that contains 2 kg of N2 at 25C and...Ch. 13.3 - Prob. 43PCh. 13.3 - Prob. 44PCh. 13.3 - Prob. 45PCh. 13.3 - Is the total internal energy of an ideal-gas...Ch. 13.3 - Prob. 47PCh. 13.3 - Prob. 48PCh. 13.3 - Prob. 49PCh. 13.3 - Prob. 50PCh. 13.3 - The volumetric analysis of a mixture of gases is...Ch. 13.3 - A mixture of nitrogen and carbon dioxide has a...Ch. 13.3 - The mass fractions of a mixture of gases are 15...Ch. 13.3 - A mixture of gases consists of 0.1 kg of oxygen, 1...Ch. 13.3 - An insulated tank that contains 1 kg of O2at 15C...Ch. 13.3 - An insulated rigid tank is divided into two...Ch. 13.3 - Prob. 59PCh. 13.3 - A mixture of 65 percent N2 and 35 percent CO2...Ch. 13.3 - Prob. 62PCh. 13.3 - Prob. 63PCh. 13.3 - Prob. 66PCh. 13.3 - Prob. 67PCh. 13.3 - Prob. 68PCh. 13.3 - Prob. 69PCh. 13.3 - The gas passing through the turbine of a simple...Ch. 13.3 - Prob. 71PCh. 13.3 - A pistoncylinder device contains 6 kg of H2 and 21...Ch. 13.3 - Prob. 73PCh. 13.3 - Prob. 74PCh. 13.3 - Prob. 75PCh. 13.3 - Prob. 76PCh. 13.3 - Prob. 77PCh. 13.3 - Prob. 78PCh. 13.3 - Prob. 79PCh. 13.3 - Prob. 81PCh. 13.3 - Fresh water is obtained from seawater at a rate of...Ch. 13.3 - Is it possible for an adiabatic liquid-vapor...Ch. 13.3 - Prob. 84PCh. 13.3 - Prob. 85RPCh. 13.3 - The products of combustion of a hydrocarbon fuel...Ch. 13.3 - A mixture of gases is assembled by first filling...Ch. 13.3 - Prob. 90RPCh. 13.3 - Prob. 91RPCh. 13.3 - Prob. 92RPCh. 13.3 - A rigid tank contains a mixture of 4 kg of He and...Ch. 13.3 - A spring-loaded pistoncylinder device contains a...Ch. 13.3 - Prob. 95RPCh. 13.3 - Reconsider Prob. 1395. Calculate the total work...Ch. 13.3 - Prob. 97RPCh. 13.3 - Prob. 100RPCh. 13.3 - Prob. 101RPCh. 13.3 - Prob. 102FEPCh. 13.3 - An ideal-gas mixture whose apparent molar mass is...Ch. 13.3 - An ideal-gas mixture consists of 2 kmol of N2and 4...Ch. 13.3 - Prob. 105FEPCh. 13.3 - Prob. 106FEPCh. 13.3 - An ideal-gas mixture consists of 3 kg of Ar and 6...Ch. 13.3 - Prob. 108FEPCh. 13.3 - Prob. 109FEPCh. 13.3 - Prob. 110FEPCh. 13.3 - Prob. 111FEP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Solve this problem and show all of the workarrow_forwardaversity of Baoyion aculty of Engineering-AIMusyab Automobile Eng. Dep. Year: 2022-2023, st Course, 1st Attempt Stage: 3rd Subject: Heat Transfer I Date: 2023\01\23- Monday Time: 3 Hours Q4: A thick slab of copper initially at a uniform temperature of 20°C is suddenly exposed to radiation at one surface such that the net heat flux is maintained at a constant value of 3×105 W/m². Using the explicit finite-difference techniques with a space increment of Ax = = 75 mm, determine the temperature at the irradiated surface and at an interior point that is 150 mm from the surface after 2 min have elapsed. Q5: (12.5 M) A) A steel bar 2.5 cm square and 7.5 cm long is initially at a temperature of 250°C. It is immersed in a tank of oil maintained at 30°C. The heat-transfer coefficient is 570 W/m². C. Calculate the temperature in the center of the bar after 3 min. B) Air at 90°C and atmospheric pressure flows over a horizontal flat plate at 60 m/s. The plate is 60 cm square and is maintained at a…arrow_forwardUniversity of Baby on Faculty of Engineering-AIMusyab Automobile Eng. Dep. Year: 2022-2023. 1 Course, 1" Attempt Stage 3 Subject Heat Transfer I Date: 2023 01 23- Monday Time: 3 Hours Notes: Q1: • • Answer four questions only Use Troles and Appendices A) A flat wall is exposed to an environmental temperature of 38°C. The wall is covered with a layer of insulation 2.5 cm thick whose thermal conductivity is 1.4 W/m. C, and the temperature of the wall on the inside of the insulation is 315°C. The wall loses heat to the environment by convection. Compute the value of the convection heat-transfer coefficient that must be maintained on the outer surface of the insulation to ensure that the outer-surface temperature does not exceed 41°C. B) A vertical square plate, 30 cm on a side, is maintained at 50°C and exposed to room air at 20°C. The surface emissivity is 0.8. Calculate the total heat lost by both sides of the plate. (12.5 M) Q2: An aluminum fin 1.5 mm thick is placed on a circular tube…arrow_forward
- Solve this and show all of the workarrow_forwardNeed helparrow_forwardY F1 α В X F2 You and your friends are planning to move the log. The log. needs to be moved straight in the x-axis direction and it takes a combined force of 2.9 kN. You (F1) are able to exert 610 N at a = 32°. What magnitude (F2) and direction (B) do you needs your friends to pull? Your friends had to pull at: magnitude in Newton, F2 = direction in degrees, ẞ = N degarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Thermodynamics - Chapter 3 - Pure substances; Author: Engineering Deciphered;https://www.youtube.com/watch?v=bTMQtj13yu8;License: Standard YouTube License, CC-BY