
EBK ORGANIC CHEMISTRY-STUD.SOLNS.MAN+SG
4th Edition
ISBN: 9781119659525
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13.10, Problem 19ATS
Interpretation Introduction
Interpretation: The product formed in the given reaction and its mechanism needs to be identified.
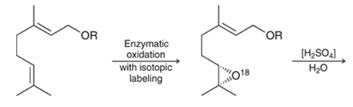
Concept Introduction:
In the acid-catalyzed reaction of an
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the structure of the product of the reaction given the IR and MS data.
Spectral analysis of the product reveals:
MS: M 150, M-15, M-43
CH.COCI
AICI,
IR: 3150-3000 cm, 2950-2850 cm
and 1700 cm
Part II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic.
a)
HO
b)
Bri
H
HH
c)
d)
H
H H Br
0
None
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY-STUD.SOLNS.MAN+SG
Ch. 13.2 - Prob. 1LTSCh. 13.2 - Prob. 1PTSCh. 13.2 - Prob. 2PTSCh. 13.2 - Prob. 3ATSCh. 13.4 - Prob. 4CCCh. 13.5 - Prob. 2LTSCh. 13.5 - Prob. 5PTSCh. 13.5 - Prob. 6ATSCh. 13.5 - Prob. 7CCCh. 13.5 - Prob. 8CC
Ch. 13.5 - Prob. 9CCCh. 13.6 - Prob. 10CCCh. 13.7 - Prob. 11CCCh. 13.7 - Prob. 12CCCh. 13.8 - Prob. 3LTSCh. 13.8 - Prob. 13PTSCh. 13.8 - Prob. 14ATSCh. 13.9 - Prob. 15CCCh. 13.10 - Prob. 4LTSCh. 13.10 - Prob. 17ATSCh. 13.10 - Prob. 5LTSCh. 13.10 - Prob. 19ATSCh. 13.11 - Prob. 20CCCh. 13.12 - Prob. 6LTSCh. 13.12 - Prob. 7LTSCh. 13 - Prob. 26PPCh. 13 - Prob. 27PPCh. 13 - Prob. 28PPCh. 13 - Prob. 29PPCh. 13 - Prob. 30PPCh. 13 - Prob. 31PPCh. 13 - Prob. 32PPCh. 13 - Prob. 33PPCh. 13 - Prob. 34PPCh. 13 - Prob. 35PPCh. 13 - Prob. 36PPCh. 13 - Prob. 37PPCh. 13 - Prob. 38PPCh. 13 - Prob. 39PPCh. 13 - Prob. 40PPCh. 13 - Prob. 41PPCh. 13 - Prob. 42PPCh. 13 - Prob. 43PPCh. 13 - Prob. 44PPCh. 13 - Prob. 45PPCh. 13 - Prob. 46ASPCh. 13 - Prob. 47ASPCh. 13 - Prob. 48ASPCh. 13 - Prob. 49ASPCh. 13 - Prob. 50ASPCh. 13 - Prob. 51ASPCh. 13 - Prob. 52ASPCh. 13 - Prob. 53ASPCh. 13 - Prob. 54IPCh. 13 - Prob. 59IPCh. 13 - Prob. 60IPCh. 13 - Prob. 61IPCh. 13 - Prob. 62IPCh. 13 - Prob. 63IPCh. 13 - Prob. 64IPCh. 13 - Prob. 65IPCh. 13 - Prob. 66IPCh. 13 - Prob. 69IPCh. 13 - Prob. 70IPCh. 13 - Prob. 71IPCh. 13 - Prob. 72IPCh. 13 - Prob. 73IPCh. 13 - Prob. 74IPCh. 13 - Prob. 77CPCh. 13 - Prob. 79CPCh. 13 - Prob. 80CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose the option that is decreasing from biggest to smallest. Group of answer choices: 100 m, 10000 mm, 100 cm, 100000 um, 10000000 nm 10000000 nm, 100000 um, 100 cm, 10000 mm, 100 m 10000000 nm, 100000 um, 10000 mm, 100 cm, 100 m 100 m, 100 cm, 10000 mm, 100000 um, 10000000 nmarrow_forwardQ1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardWhich is NOT the typical size of a bacteria? 1000 nm 0.001 mm 0.01 mm 1 umarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY