
Concept explainers
(a)
Interpretation:
The IUPAC name of the following compound should be determined.
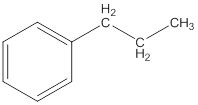
Concept Introduction:
When the mixture of hydrocarbons from natural resources such as coal or petroleum are separated, certain compounds are emerged with pleasant odors and are thus known as
Hydrocarbons with sigma bonds and delocalization of pi electrons between carbon atoms, thus forms a circle is said to be aromatic hydrocarbons.
Rules of naming aromatic compounds are:
- Aromatic compounds are named as a benzene derivative.
- For single substituent, the benzene ring is symmetrical. Thus, all the positions are equivalent.
- For more than one substituent, the benzene ring is not symmetrical. Thus, numbering will be done from one substituent which is attached to ring to other substituent through shorter path. Numbering should be done in a way that the first substituent gets the lowest number.
- In naming, the names of substituents are listed in alphabetical order.
(b)
Interpretation:
The IUPAC name of the following compound should be determined.
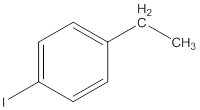
Concept Introduction:
When the mixture of hydrocarbons from natural resources such as coal or petroleum are separated, certain compounds are emerged with pleasant odors and are thus known as aromatic hydrocarbons.
Hydrocarbons with sigma bonds and delocalization of pi electrons between carbon atoms, thus forms a circle is said to be aromatic hydrocarbons.
Rules of naming aromatic compounds are:
- Aromatic compounds are named as a benzene derivative.
- For single substituent, the benzene ring is symmetrical. Thus, all the positions are equivalent.
- For more than one substituent, the benzene ring is not symmetrical. Thus, numbering will be done from one substituent which is attached to ring to other substituent through shorter path. Numbering should be done in a way that the first substituent gets the lowest number.
- In naming, the names of substituents are listed in alphabetical order.
(c)
Interpretation:
The IUPAC name of the following compound should be determined.
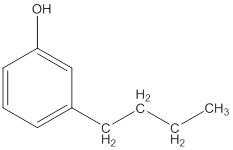
Concept Introduction:
When the mixture of hydrocarbons from natural resources such as coal or petroleum are separated, certain compounds are emerged with pleasant odors and are thus known as aromatic hydrocarbons.
Hydrocarbons with sigma bonds and delocalization of pi electrons between carbon atoms, thus forms a circle is said to be aromatic hydrocarbons.
Rules of naming aromatic compounds are:
- Aromatic compounds are named as a benzene derivative.
- For single substituent, the benzene ring is symmetrical. Thus, all the positions are equivalent.
- For more than one substituent, the benzene ring is not symmetrical. Thus, numbering will be done from one substituent which is attached to ring to other substituent through shorter path. Numbering should be done in a way that the first substituent gets the lowest number.
- In naming, the names of substituents are listed in alphabetical order.
(d)
Interpretation:
The IUPAC name of the following compound should be determined.
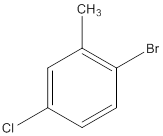
Concept Introduction:
When the mixture of hydrocarbons from natural resources such as coal or petroleum are separated, certain compounds are emerged with pleasant odors and are thus known as aromatic hydrocarbons.
Hydrocarbons with sigma bonds and delocalization of pi electrons between carbon atoms, thus forms a circle is said to be aromatic hydrocarbons.
Rules of naming aromatic compounds are:
- Aromatic compounds are named as a benzene derivative.
- For single substituent, the benzene ring is symmetrical. Thus, all the positions are equivalent.
- For more than one substituent, the benzene ring is not symmetrical. Thus, numbering will be done from one substituent which is attached to ring to other substituent through shorter path. Numbering should be done in a way that the first substituent gets the lowest number.
- In naming, the names of substituents are listed in alphabetical order.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
- Can you please help me with drawing the Lewis structure of each molecular formula?I truly appreciate you!arrow_forwardPlease draw and explainarrow_forwardDescribe each highlighted bond in terms of the overlap of atomic orbitals. (a) Н Н H H [References] HIC H H C H H-C-CC-N: H σ character n character (b) HIC H H H H-C-C-C HIC H Н H O-H σ character n character Submit Answer Try Another Version 3 item attempts remainingarrow_forward
- 11 Naming and drawing alcohols Write the systematic (IUPAC) name for each of the following organic molecules: structure OH HO OH Explanation Check name ☐arrow_forwardwhat is the drawn mechanism for diethyl carbonate and 4 - bromo - N, N -dimethylaniline to create crystal violet?arrow_forwardWhich of the following compounds are constitutional isomers of each other? I and II O II and III O III and IV OI and IV O II and IV CI H CI H CI H H CI H-C-C-CI C-C-C-CI H-C-C-CI H-C-C-CI H CI Ĥ ĆI A A Ĥ ĆI || IVarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardQ1: Curved Arrows, Bronsted Acids & Bases, Lewis Acids & Bases Considering the following reactions: a) Predict the products to complete the reactions. b) Use curved electron-pushing arrows to show the mechanism for the reaction in the forward direction. Redraw some of the compounds to explicitly illustrate all bonds that are broken and all bonds that are formed. c) Label Bronsted acids and bases in the left side of the reactions. Label conjugate acids and bases in the right side of the reactions. d) Label Lewis acids and bases, nucleophiles and electrophiles in the left side of the reactions. A. + OH CH30: OH B. + HBr C. H₂SO4 D. CF 3. CH 3 + HCI N H fluoxetine antidepressant 1↓ JDownloadarrow_forwardDon't used Ai solutionarrow_forward
- Part 3: AHm,system Mass of 1.00 M HCI Vol. of 1.00 M HCI Mass of NaOH(s) Total Mass in Calorimeter Mole product if HCI limiting reactant Trial 1 62.4009 1.511g Mole product if NaOH limiting reactant Limiting reactant Initial Temperature Final Temperature 23.8°C 37.6°C Change in Temperature AHm,system (calculated) Average AHm,system (calculated) (calculated) (calculated) Trial 2 64.006g 1.9599 (calculated) (calculated) (calculated) (calculated) (calculated) (calculated) 24.7°C 41.9°C (calculated) (calculated) (2 pts. each)arrow_forwardDon't used Ai solutionarrow_forwardWhat is the numerical value of the slope using the equation y=-1.823x -0.0162 please show calculationsarrow_forward
