
Concept explainers
(a)
Interpretation:
Skeletal structures of molecule A should be drawn by showing the stereochemistry at the carbon-carbon double bond.
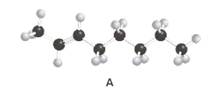
Concept Introduction:
The skeletal structure of a molecule is the formulaic representation excluding hydrogens.
(b)
Interpretation:
Stereoisomer of structure A should be drawn.
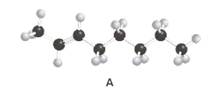
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Isomers are the different molecules that have the same molecular formula but differ in molecular arrangement in the space. There are several groups of isomers.
Stereoisomers are the form of isomers which have different three-dimensional orientations in the space.
(c)
Interpretation:
Constitutional isomer of structure A should be drawn.
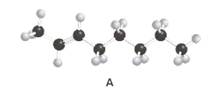
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Isomers are the different molecules that have the same molecular formula but differ in molecular arrangement in the space. There are several groups of isomers.
Constitutional isomers are the form of isomers which have atoms bonded to each other in different ways.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
- The following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forward
- How many chiral centers/stereocenters are there in the following molecule? 1 2 3 4arrow_forwardWhich of these correspond to the molecule: 2,5-dimethylheptanearrow_forwardGiven the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





