
Concept explainers
(a)
Interpretation:
Structure of the molecule B should be converted to a condensed structure and IUPAC name of the molecule should be given.
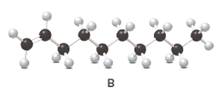
= (
Concept Introduction:
In alkene nomenclature longest C chain will be considered as the parent/ main C chain. At the end of the
Answer to Problem 96P
Condensed structure -
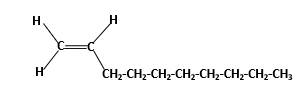
IUPAC name −Decene
Explanation of Solution
In alkenes there is a carbon-carbon double bond and different groups are connected to the two carbons. In most of the alkenes; out of four binding positions of carbon-carbon double bond, two of them are binds with two hydrogen atoms and other two binding positions are binds with two different alkyl groups.In this molecule, carbon atoms are represented in black and hydrogen atoms are represented in white color.Structure of a chemical compound is the arrangement of atoms and bonds in the space.
Condensed structure -
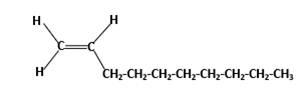
In this molecule, main carbon chain which contains the carbon-carbon double bond is a ten membered chain and there are no substituents in this molecule. According to the numbering of the main chain double bond gets the lowest number and it is number 1.Hence, IUPAC name of the molecule is decene.
(b)
Interpretation:
The resulting product should be identified after reacting
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 96P
Explanation of Solution
Unsaturated (
(c)
Interpretation:
The resulting product should be identified after reacting
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 96P
Explanation of Solution
Unsaturated (
Refer to the below reaction;
(d)
Interpretation:
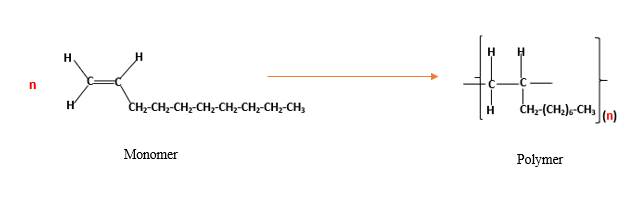
The resulting polymer should be identified after
Concept Introduction:
Alkenes are hydrocarbon molecules that contain a carbon-carbon double bond inside a molecule.
Answer to Problem 96P
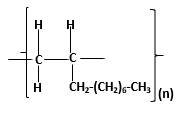
Explanation of Solution
Polymers are high molecular weight macromolecules which formed from covalently bonded monomer molecules. When forming polymers from alkene monomers, the weak bond which is in the carbon-carbon double bond is broken and new strong bond will form to connect the monomer molecules together. Hence; weak bond of the carbon-carbon double bond of
Want to see more full solutions like this?
Chapter 13 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
- Polymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forwardDraw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forward
- One of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forwardDraw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forward
- curved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forwardIdentify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forwardH- H H H H H H Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H H H A. H H H H-C CI H H D. H H H H H H C C -H H C C H H H H B. H CI H H- C C H H H H E. H CI H C.arrow_forward
- Why doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?arrow_forward6. A solution (0.0004 M) of Fe(S2CNEt2)3 (see the structural drawing below) in chloroform has absorption bands at: 350 nm (absorbance A = 2.34); 514 nm(absorbance A = 0.0532); Calculate the molar absorptivity values for these bands. Comment on their possible nature (charge transfer transitions or d-d S N- transitions?). (4 points)arrow_forwardWhat is the mechanism for this?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
