
Concept explainers
The following table lists the concentrations of the principal ions in seawater:
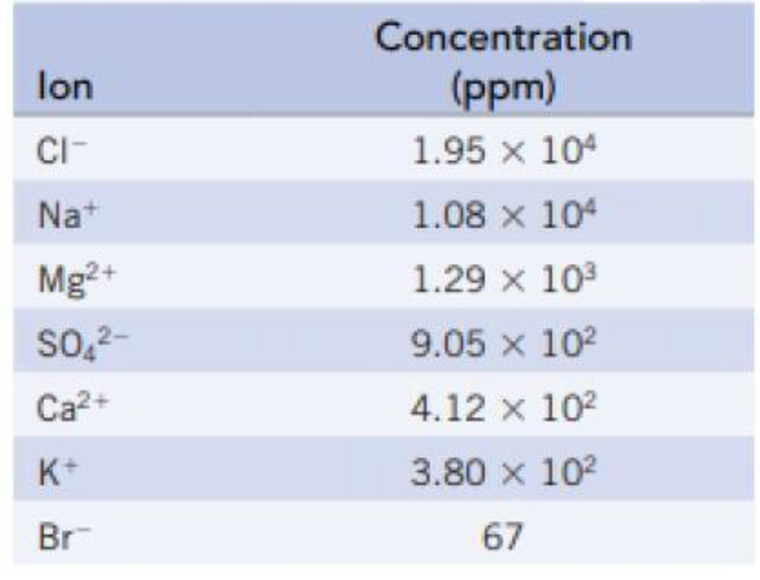
- (a) Calculate the freezing point of seawater.
- (b) Calculate the osmotic pressure of seawater at 25 °C. What is the minimum pressure needed to purify seawater by reverse osmosis?
(a)
Interpretation: The freezing point of seawater has to be determined.
Concept introduction:
Colligative properties: Properties of solutions which having influence on the concentration of the solute in it. Colligative properties are,
- Decrease in the vapor pressure
- Increase in the boiling point
- Decline in the freezing point
- Osmotic pressure
Freezing point depression: The freezing point of the solution varies with the solute concentration.
The number of moles of any substance can be determined using the equation
Answer to Problem 79GQ
Freezing point of seawater is
Explanation of Solution
Given,
Molal freezing point depression constant of water is
The value
Hence, the concentration given in ppm can be taken as the mass of each of the ions on
The number of moles of any substance can be determined using the equation
Number of moles of
Number of moles of
Number of moles of
Number of moles of
Number of moles of
Number of moles of
Number of moles of
So the total moles of principle ions in
Molality of seawater is,
Depression in freezing point is,
Therefore,
Freezing point of seawater is,
Freezing point of seawater is
(b)
Interpretation: The osmotic pressure of seawater at
Concept introduction:
Colligative properties: Properties of solutions which having influence on the concentration of the solute in it. Colligative properties are,
- Decrease in the vapor pressure
- Increase in the boiling point
- Decline in the freezing point
- Osmotic pressure
Osmotic pressure: The pressure created by the column of solution for the system at equilibrium is a measure of the osmotic pressure and is calculated by using the equation,
where,
c is the molar concentration
The number of moles of any substance can be determined using the equation
Answer to Problem 79GQ
The osmotic pressure of seawater at
Explanation of Solution
Given,
The molarity of the solute is
The osmotic pressure of seawater is,
The osmotic pressure of seawater at
To purify the seawater using reverse osmosis method, there should be a minimum pressure of
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry & Chemical Reactivity, Hybrid Edition (with OWLv2 24-Months Printed Access Card)
Additional Science Textbook Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Physical Science
Human Biology: Concepts and Current Issues (8th Edition)
Campbell Biology (11th Edition)
Organic Chemistry
- What is the complete reaction mechanism for the chlorination of Ethane, C2H6?arrow_forwardA 13C NMR spectrum is shown for a molecule with the molecular formula of C6H100. Draw the structure that best fits this data. 220 200 180 160 140 120100 80 60 40 20 Drawingarrow_forwardPlease help me figure out the blan areas with step by step calculations.arrow_forward
- needing help draw all of the possible monochlorination products that would result from the free radical chlorination of 2,3,4-trimethylpentanearrow_forwardHAND DRAWarrow_forwardBased on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10. Provide assignment for the provided structurearrow_forward
- O Predict the 'H NMR integration ratio for the following structure. IV I. 3 H A II. 1 H III. 2 H IV. 3 H I. 3 H B II. O H III. 2 H IV. 3 H I. 3 H C II. 2 H III. 2 Harrow_forward205. From the definition of the Gibbs free energy, G = H - TS, derive the Gibbs-Helmholtz equation a (or (G)),- =- H T2arrow_forward229. Show that ән (~~)--(*), др =V-T Parrow_forward
- Describe hyperconjugation (Organic Chemistry).arrow_forwardDescribe the mesomeric or resonance effect and differentiate between types +E or +M and -R or -M.arrow_forwardI need help with the following two problems, understanding them in a simple manner. Can you please draw them out for me with a detailed explanation so that I can better comprehend? I'm a visual person, so I definitely need that. Thank you very much!arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





