
A solution of benzoic acid in benzene has a freezing point of 3.1 °C and a boiling point of 82.6 °C. (The freezing point of pure benzene is 5.50 °C, and its boiling point is 80.1 °C) The structure of benzoic acid is
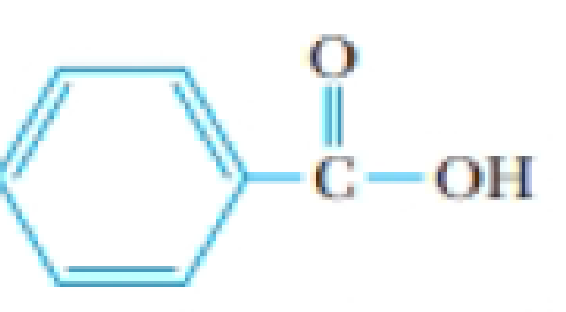
Benzoic acid, C6H5CO2H
What can you conclude about the state of the benzoic acid molecules at the two different temperatures? Recall the discussion of hydrogen bonding in Section 11.3.
Trending nowThis is a popular solution!

Chapter 13 Solutions
Chemistry & Chemical Reactivity, Hybrid Edition (with OWLv2 24-Months Printed Access Card)
Additional Science Textbook Solutions
Microbiology Fundamentals: A Clinical Approach
Organic Chemistry
MARINE BIOLOGY
Applications and Investigations in Earth Science (9th Edition)
Campbell Essential Biology (7th Edition)
Essentials of Human Anatomy & Physiology (12th Edition)
- A sample of sulfur weighing 0.210 g was dissolved in 17.8 g of carbon disulfide, CS2 ( Kb=2.43 C/m). If the boiling point elevation was 0.107 C, what is the formula of a sulfur molecule in carbon disulfide?arrow_forwardUse molecular structures and noncovalent interactions to explain why dimethyl ether, (CH3)2O, is completely miscible in water, but dimethylsulfide, (CH3)2S, is only slightly water soluble.arrow_forwardDescribe the formation of hydrogen bonds in propanol, CH3CH2CH2OH. Represent possible hydrogen bonding structures in propanol by using structural formulas and the conventional notation for a hydrogen bond.arrow_forward
- 8.52 Rank the following hydrocarbons in order of increasing vapor pressure: C2H6,C10H22,CH4,C7H16,C22H46 .arrow_forwardPotassium nitrate (KNO3) crystals were insoluble in water at room temperature. Heating the solution increases the solubility of the crystals in water. Briefly explain this phenomenon based on intermolecular forces of attraction of molecules.arrow_forwardThe molecular mass of butanol, C4H,OH, is 74.14; that of ethylene glycol, CH2(OH)CH,OH, is 62.08, yet their boiling points are 117.2 °C and 174 °C, respectively. Explain the reason for the difference. The two hydroxyl groups in ethylene glycol provide more locations for the formation of hydrogen bonds. The existence of more hydrogen bonds considerably decreases the boiling point O The two hydroxyl groups in ethylene glycol provide less locations for the formation of hydrogen bonds. The existence of less hydrogen bonds considerably increases the boiling point The two hydroxyl groups in ethylene glycol provide more locations for the formation of hydrogen bonds. The existence of more hydrogen bonds considerably increases the boiling pointarrow_forward
- What phenomenon does hydrogen bonding in water create to enable certain insects to walk on puddles and other water surfaces? Would you expect liquid ammonia to produce this phenomenon to the same extent? Why (not)? Assuming these insects wouldn’t be bothered by the much lower temperature, would you expect their attempts to walk on liquid ammonia to be successful?arrow_forward15.arrow_forwardArrange HCI, H0, SiH, in order of increasing boiling point temperature: O HCI < SIH4 < H2O O SIIH, < HCI < H;O O SiH, « H2O < HCIarrow_forward
- Solubility- hexachlorobenzene Q1)Using the Lewis structures of the molecule and water, identify and label areas of intermolecular attraction. ▪More than one water molecule should be shown ▪Label the type of IM forces present b)Using the Lewis structures of the molecule and a nonpolar solvent (e.g., hexane), identify and label areas of intermolecular attraction. c)explain the solubility (or lack thereof) of your molecule in water. When available, use solubility data (at the same temperature) to support your explanation. d)explain the solubility (or lack thereof) of your molecule in nonpolar solvents. When available, use solubility data (at the same temperature) to support your explanation. e)when dissolved in water, qualitatively discuss whether the molecule is a strong electrolyte, weak electrolyte, or nonelectrolyte. Please answer very soon will give rating surely All questions answers neededarrow_forwardC. Explain the following occurrences in terms of molecular polarity and intermolecular forces. 1. Propane can be liquefied, the reason why it is produced as liquefied petroleum gas. (a) What intermolecular force is present between propane gases that makes them attract one another and be liquefied? (b) As soon as we open our gas stove, it is immediately converted back to gas state. Explain it in terms of the strength of the intermolecular force present between propane molecules.arrow_forwardMineral oil consists of a mixture of hydrocarbons (compounds consisting of just carbon and hydrogens) consisting of nine or more carbon atoms. An example of a large hydrocarbon is eicosane which has a molecular formula of C20H42. The boiling point of eiconsane is 343.1 oC. Why is the boiling point of this nonpolar molecule so much higher than the boiling point of water (a polar molecule with a boiling point of 100 oC). In your answer, be sure and include the role of specific intermolecular attractive forces for each molecule.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





