
Concept explainers
Interpretation:
The compound, which reacts faster with sodium methoxide in methanol in each of the given pair of compounds is to be determined and the chemical equation for the faster reaction is to be written.
Concept introduction:
Nucleophilic
In nucleophilic aromatic substitution reactions, the nucleophile substitutes a leaving group from the aryl ring.
Aryl halides bearing an electron withdrawing substituent undergo nucleophilic substitution rapidly.
The substituents attached ortho and para with respect to the halogen atom in the aryl halide react at similar rates. The substituents attached at meta position in the aryl halide react at slower rates than ortho and para substituents.
Electron withdrawing substituents stabilize the intermediate carbanion formed and thus are strongly activating substituents in the nucleophilic aromatic substitution reactions.
Electron donating substituents destabilize the intermediate carbanion formed and thus are strongly deactivating substituents in the nucleophilic aromatic substitution reactions.
Answer to Problem 43P
Solution:
a)
The reaction is as follows:
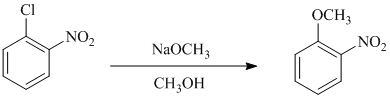
b) In between
The reaction is as follows:

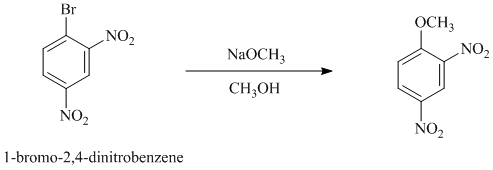
c) In between
The reaction is as follows:
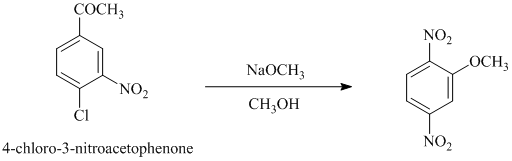
d) In between
The reaction is as follows:
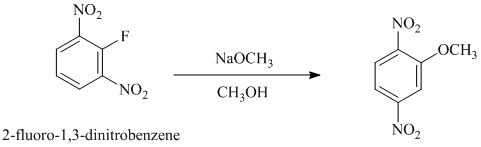
e) In between
The reaction is as follows:
Explanation of Solution
a)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
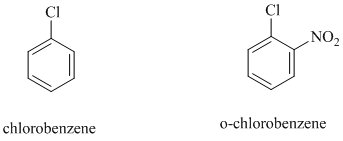
In chlorobenzene, a chlorine atom is attached to the benzene ring while in
The reaction of
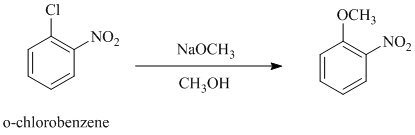
b)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
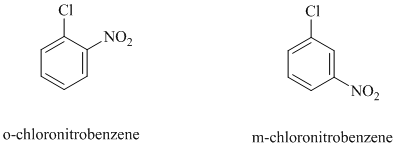
In both the given aryl halides, a strong electron withdrawing substituent is attached on the ring. In
The reaction of
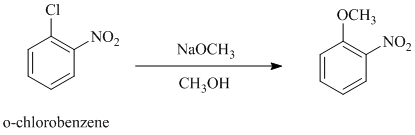
c)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
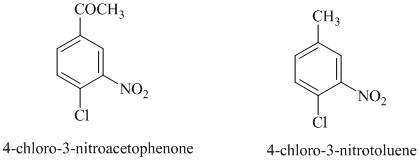
In
In
Thus, in between
The reaction of

d)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
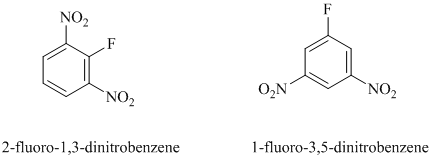
Nitro substituents are strong electron withdrawing substituents.
In
Electron withdrawing substituents at ortho and para positions activate the ring more than the electron withdrawing substituents at meta positions.
Thus, in between
The reaction of

e)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
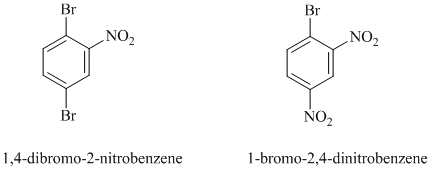
In
The reaction of

Want to see more full solutions like this?
Chapter 13 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- Please predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forward
- If we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forwarddrawing, no aiarrow_forwardIf CH3COCH2CH(OCH3)2 (4,4-dimethoxy-2-butanone) and hydrazine react, two isomeric products are formed. State their structure and which will be the majority.arrow_forward
- + Reset Provide the correct IUPAC name for the compound shown here. 4-methylhept-2-ene (Z)- (E)- 1-6-5-2-3-4- cyclo iso tert- sec- di tri hept hex oct meth eth pent ane yne ene ylarrow_forward+ Provide the correct IUPAC name for the compound shown here. Reset H3C H H C CH3 CH-CH3 1-3-methylpent ene trans- cis- 5-6-3-1-2-4- tert- tri sec- di cyclo iso but pent hex meth prop eth yl ane ene yne ☑arrow_forwarddrawing, no aiarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
