
Concept explainers
Write the structure of the organic product in each of the following reactions. If electrophilic
aromatic substitution occurs, assume only monosubstitution.
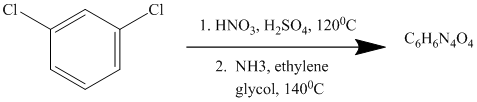
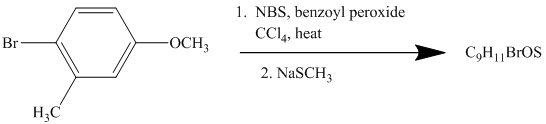
Interpretation:
Structures for the major organic product of an electrophilic aromatic monosubstituted reactions in each of the given reactions is to be written.
Concept introduction:
Ring activating groups such as hydroxide, amines, alkoxy groups etc. direct the incoming electrophile to ortho or para position with respect to their position.
Ring deactivating groups such as nitro, formyl, esters, acyl, etc. direct the incoming electrophile to meta position with respect to their position.
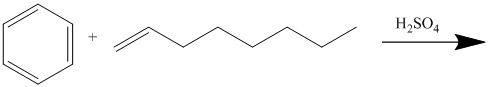
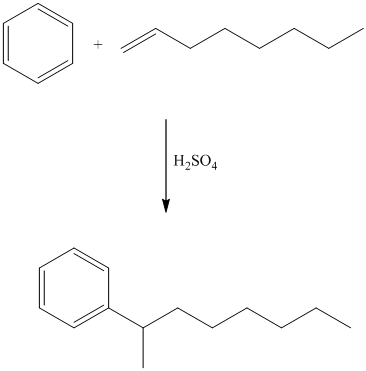
In electrophilic aromatic substitution, alkenes, which are converted to carbocations by protonation in the presence of a strong acid, can be used to alkylate benzene.
Aryl halides (halogens attached to the
Heterocyclic aromatic compounds such as pyrrole, furan, thiophene have electron rich aromatic rings and are extremely reactive towards electrophilic aromatic reactions. The incoming electrophile attaches selectively to C2 carbon atom in case of heterocyclic aromatic compounds.
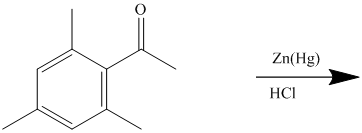
The reagent zinc amalgam and concentrated hydrochloric acid is used to convert a carbonyl group into methylene unit. This reaction is known as Clemmenson’s reduction.
Answer to Problem 33P
Solution:
Explanation of Solution
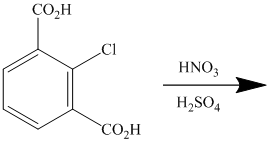
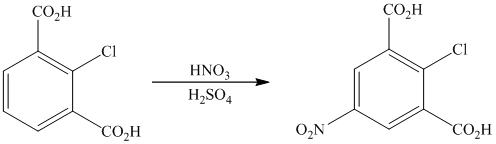
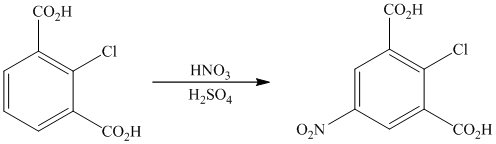
The given reactant molecule has a benzene ring with two carboxylic acid groups and one chlorine as substituents. The mixture of
The carboxylic acid groups are strong deactivating and meta directing groups. Chlorine atom slightly deactivates the ring but is ortho-para directing group. The incoming electrophile, nitronium ion, will be directed to the meta position with respect to the two carboxylic acid groups, which is also the para position with respect to the chlorine atom. Both the attached substituents reinforce electrophilic aromatic substitution at para position with respect to chlorine.
Thus, the reaction is as follows:
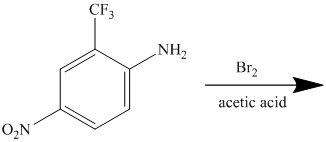
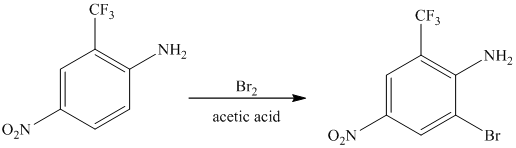
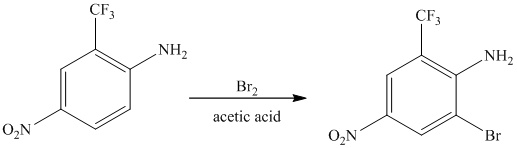
The given reactant molecule has a benzene ring attached to three substituents. The three substituents are amino group
Due to the presence of two strong deactiving substituents, the incoming electrophile is directed to the meta position with respect to
Thus, the reaction is as follows:
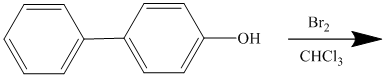
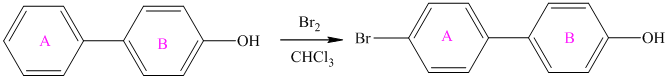
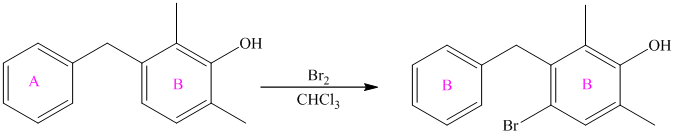
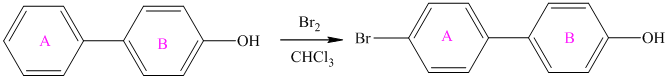
For a biphenyl compound, two benzene rings are connected to each other by a single bond. If only one substituent is present in the biphenyl ring, then its position is designated as being ortho, meta, or para with respect to the other ring.
In biphenyl, ring B is considered as a substituent of ring A. Ring B is an aryl ring with a hydroxyl group attached to it. Hydroxyl group is an activating group and is ortho-para directing. Thus, monosubstitution takes place to para position with respect to ring B.
Thus, the reaction is as follows:
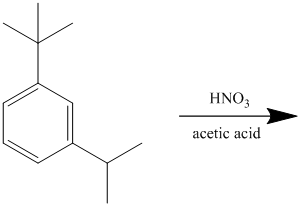
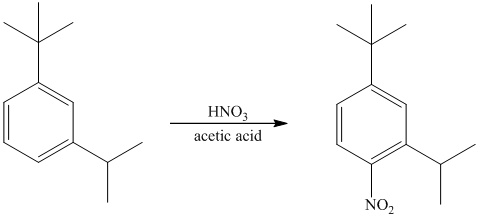
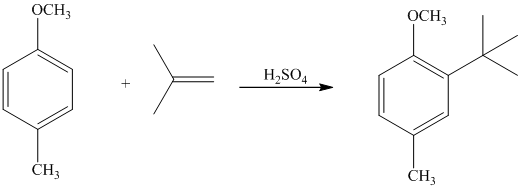
The given reactant molecule has a benzene ring attached to alkyl substituents. The one substituent is an isopropyl group while the other is tert-butyl group.
Both the substituents are strong activators and are ortho-para directors. Tert-butyl substituent is more sterically hindered than an isopropyl substituent. Thus, the electrophilic aromatic substitution takes place at ortho position with respect to the isopropy group, which is also the para position of tert-butyl group.
Thus, the reaction is as follows:
In the given reaction,
Thus, the reaction is as follows:
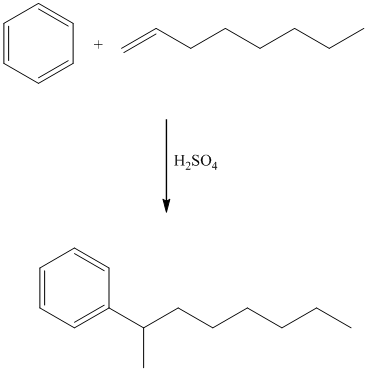
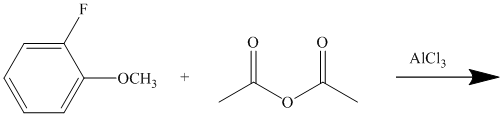
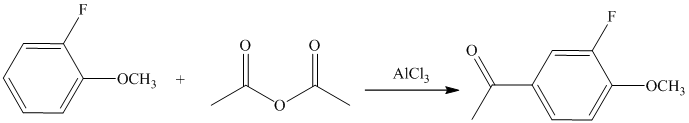
This is a Friedel Crafts acylation reaction. The benzene ring has two substituents attached –fluorine and methoxy groups. Acetic acid and aluminum chloride produce an acyl cation, which is an electrophile in this reaction.
The fluorine is a deactivator but ortho-para director. The methoxy group is a strong activator and ortho-para director. Thus, the strong activating methoxy group will direct the incoming acyl cation to its para position.
Thus, the reaction is as follows:
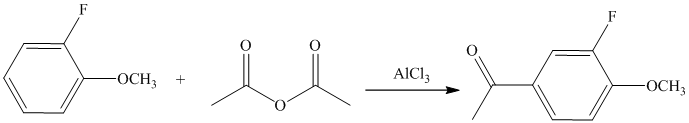
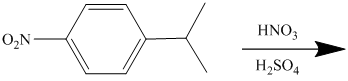
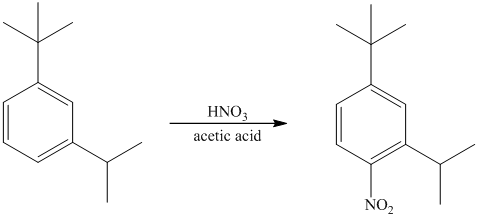
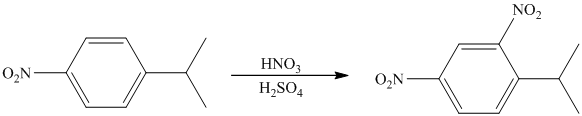
The given electrophilic aromatic substation reaction is nitration. The mixture of
The isopropyl group attached to the benzene ring is an activator and ortho-para director. The nitro group is a strong deactivator and meta director. When mononitration of this molecule takes place, the incoming electrophile is directed meta to nitro group, which is the para position with respect to the isopropyl group.
Thus, the reaction is as follows:
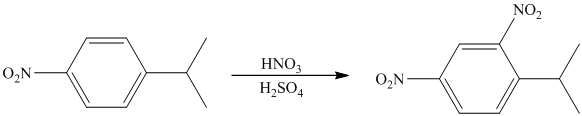
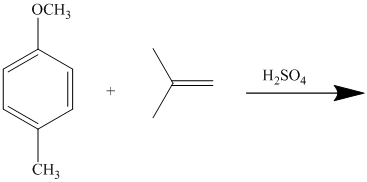
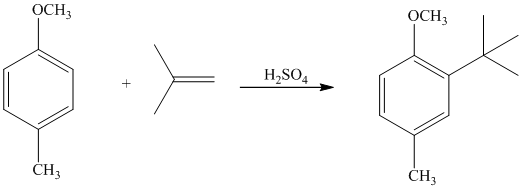
In the given reactant molecule, a benzene ring has two substituents attached. One substituent is the methoxy group while the other is methyl group. Both methyl and methoxy groups will activate the ring and are ortho-para directors.
The methoxy group activates the ring stronger than the methyl group. Thus, the alkylation takes place at ortho position with respect to methoxy group.
Thus, the reaction is as follows:
The given reactant molecule shows two benzene rings connected by a
When this compound undergoes electrophilic aromatic substitution, the incoming electrophile is directed to the para position with respect to the hydroxyl group in ring B, which is also the ortho position with respect to ring A.
The reaction is as follows:
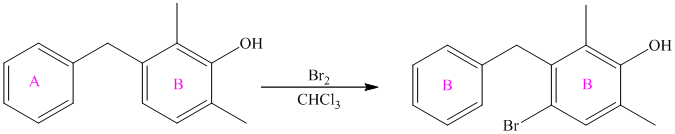
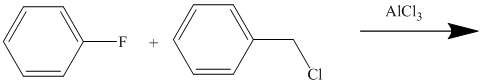
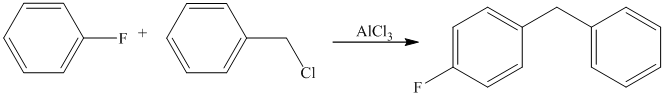
The given reactant molecule has a benzene ring attached to one fluorine while the other reactant is an benzyl chloride.
The fluoro group is a deactivator, but it will direct the aryl group in the para position. In this Friedel-Craft alkylation reaction, the benzyl group will be directed at the para position with respect to the fluorine atom.
Thus, the reaction is as follows:
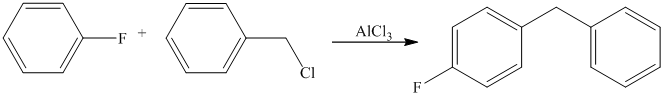
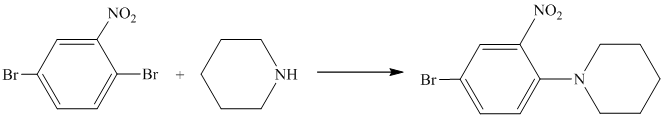
Aryl halides (halogens attached to the
Thus, the reaction is as follows:
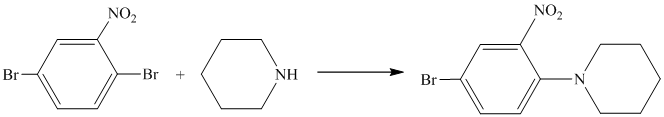
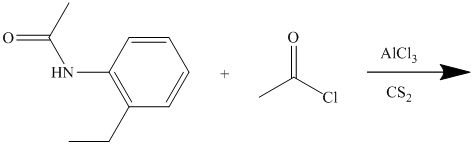
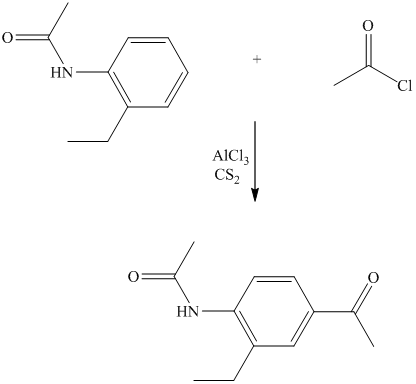
In the reactant molecule, a benzene ring is attached to two substituents. One substituent is acylamino while the other is ethyl substituent. Both these substituents are strong activators and ortho-para directors. This is a Friedel Crafts acylation reaction, in which the acyl group will add to the para position with respect to the acylamino group and ethyl group, which are strong activators.
Thus, the reaction is as follows:
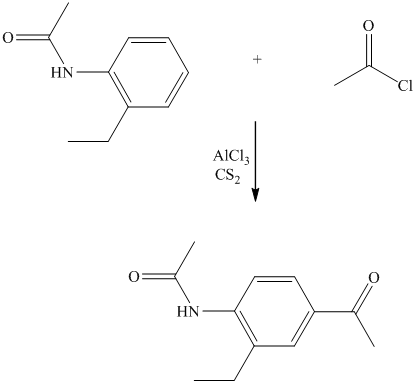
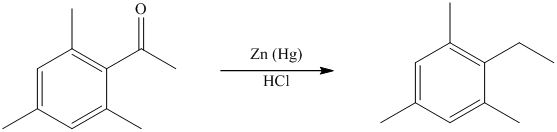
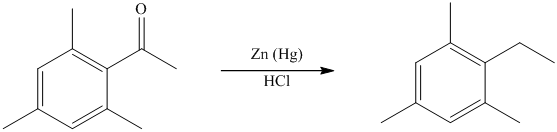
The give reactant benzene ring has four substituents. Three substituents are methyl groups while one is an acyl group. The reagent zinc amalgam and concentrated hydrochloric acid is used to convert a carbonyl group into methylene unit. This reaction is known as Clemmenson reduction.
Thus, the reaction is as follows:
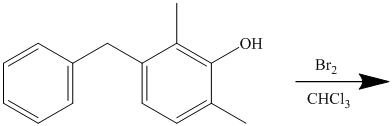
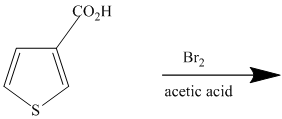
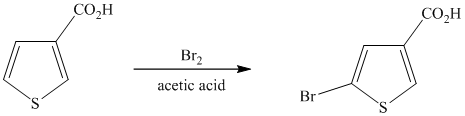
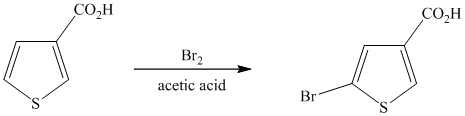
This is an example of a heterocyclic electrophilic aromatic substitution reaction. Thiophenes have electron rich aromatic rings and are extremely reactive towards electrophilic aromatic substitution, preferably at C2-C5 carbon atom in the ring. The thiophene ring has a carboxylic acid group as a substituent attached. Carboxylic acid group deactivates the ring and is a meta directing group. Thus, in bromination of substituted thiophene, the bromine will add to meta position with respect to the carboxylic acid group, which is also the C2 position of thiophene.
Thus, the reaction is as follows:
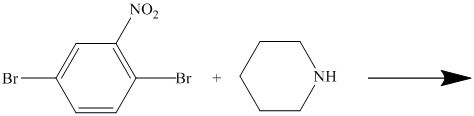
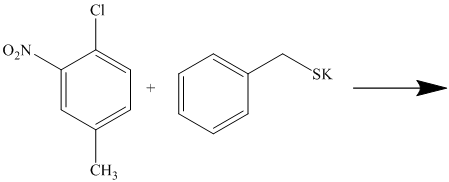
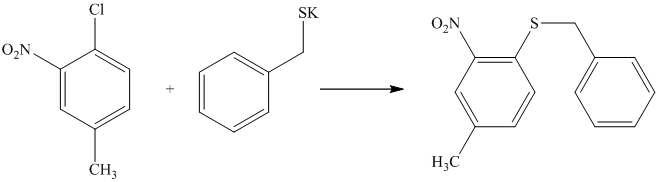
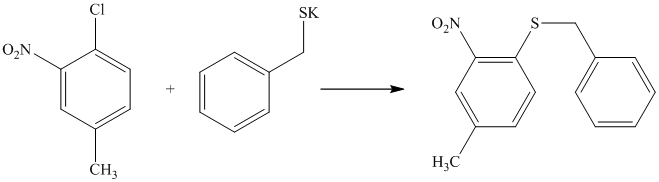
There is a strong deactivator group (nitro group) present at ortho position to the chlorine. Because of this, that chlorine will undergo nucleophilic substitution reaction. The nucleophile is sulfur, which will attack the chlorine and form the product.
Thus, the reaction is as follows:
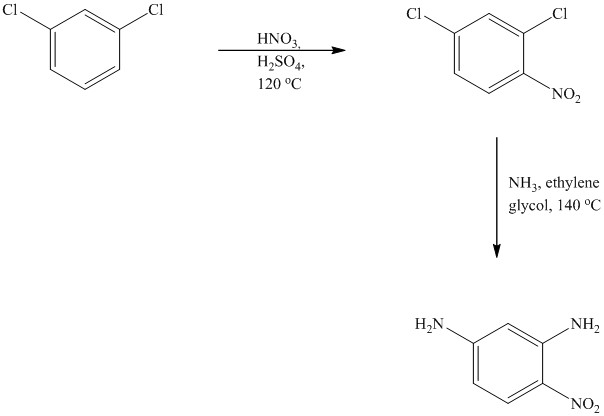
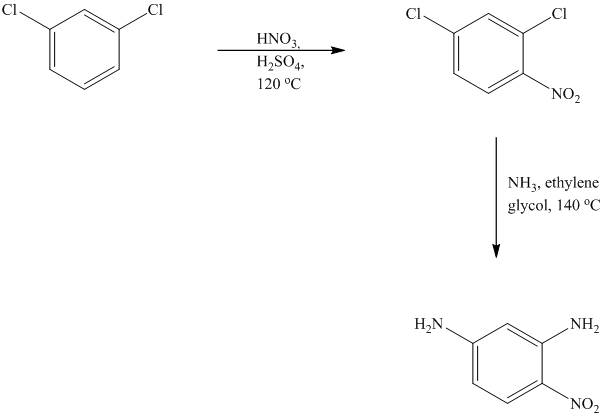
This is an example of an electrophilic aromatic substitution followed by a nucleophilic aromatic substitution. In the given reactant molecule, a benzene ring has two chloro groups, which are deactivators but ortho-para directors. The mixture of
As a first step, nitration of the given reactant molecule takes place such that the nitro group will be attached to the ortho position with respect to one of the chlorine atoms and para with respect to the other chlorine atom. Thus, in the product for this step one, there is a benzene ring with three substituents, the two chlorine atoms meta to each other and one nitro group para to one chlorine and ortho to the other.
In this product, the nitro group is placed where it is ortho to one chlorine and para to the other one. Since there is a strong deactivator group (nitro group) present at ortho and para positions to both the chlorines, the aryl chloride will undergo nucleophilic substitution reaction. The nucleophile is ammonia. Both the chlorine atoms will be replaced by amino groups.
Thus, the reaction is as follows:
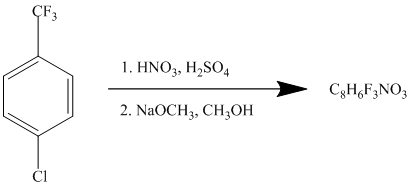
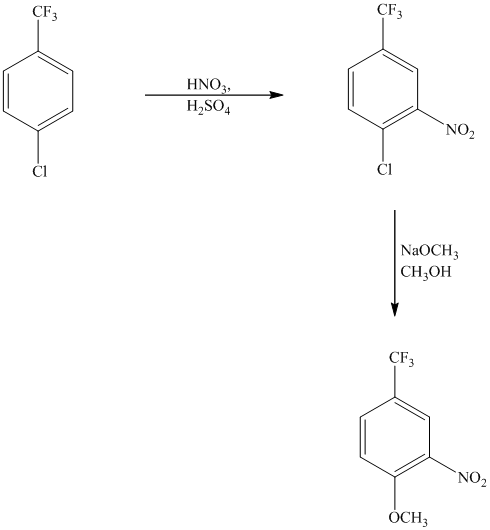
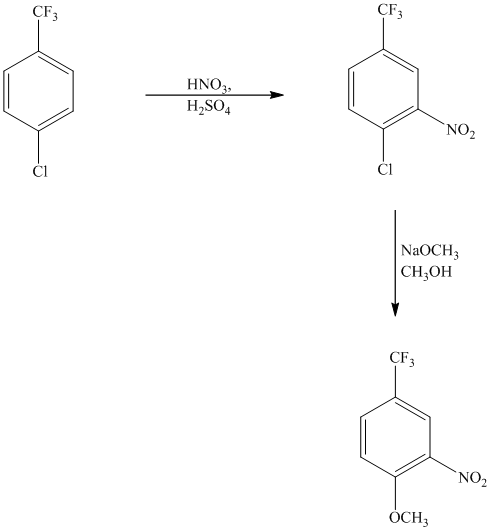
This is an example of an electrophilic aromatic substitution followed by a nucleophilic aromatic substitution. The mixture of
If the reactant molecule undergoes a nitration reaction, then the nitro group is placed ortho with respect to the chlorine atom, which is also the meta position with respect to the trifluoromethyl group.
This first step produces a compound in which two strong deactivating groups are attached at ortho and para positions with respect to the chlorine atom. Thus, a nucleophilic aromatic substitution reaction will take place. The nucleophile is methoxide, which will attack the chlorine and form the product.
Thus, the reaction is as follows:
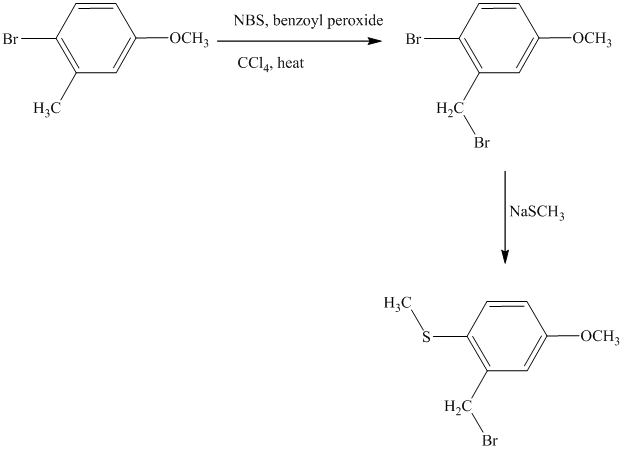
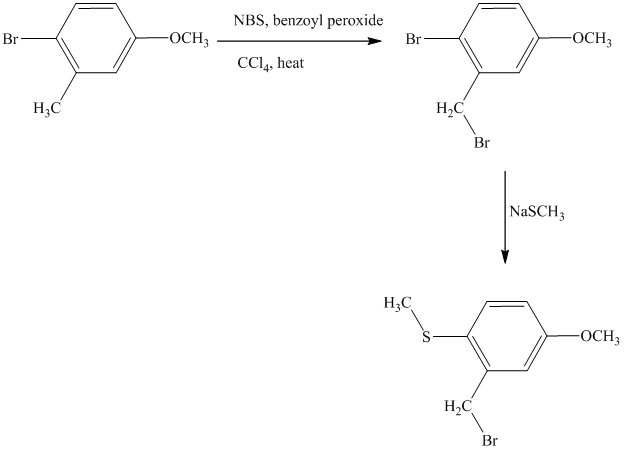
This is an example of an electrophilic aromatic substitution followed by a nucleophilic aromatic substitution. Here, NBS reagent adds bromine to benzylic carbon, so bromine will be added to the
Thus, the reaction is as follows:
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry - Standalone book
- Give reason(s) for six from the followings [using equations if possible] a. Addition of sodium carbonate to sulfanilic acid in the Methyl Orange preparation. b. What happened if the diazotization reaction gets warmed up by mistake. c. Addition of sodium nitrite in acidified solution in MO preparation through the diazotization d. Using sodium dithionite dihydrate in the second step for Luminol preparation. e. In nitroaniline preparation, addition of the acid mixture (nitric acid and sulfuric acid) to the product of step I. f. What is the main reason of the acylation step in nitroaniline preparation g. Heating under reflux. h. Fusion of an organic compound with sodium. HAND WRITTEN PLEASEarrow_forwardedict the major products of the following organic reaction: u A + ? CN Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Te LMUNDARYarrow_forwardSketch the intermediates for A,B,C & D.arrow_forward
- Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? O ? A . If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. . If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. ㅇ 80 F5 F6 A 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cente FIGarrow_forwardIn methyl orange preparation, if the reaction started with 0.5 mole of sulfanilic acid to form the diazonium salt of this compound and then it converted to methyl orange [0.2 mole]. If the efficiency of the second step was 50%, Calculate: A. Equation(s) of Methyl Orange synthesis: Diazotization and coupling reactions. B. How much diazonium salt was formed in this reaction? C. The efficiency percentage of the diazotization reaction D. Efficiency percentage of the whole reaction.arrow_forwardHand written equations pleasearrow_forward
- Hand written equations pleasearrow_forward> each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X Ś CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) © 2025 McGraw Hill LLC. All Rights Farrow_forwardNMR spectrum of ethyl acetate has signals whose chemical shifts are indicated below. Which hydrogen or set of hydrogens corresponds to the signal at 4.1 ppm? Select the single best answer. The H O HỌC—C—0—CH, CH, 2 A ethyl acetate H NMR: 1.3 ppm, 2.0 ppm, 4.1 ppm Check OA B OC ch B C Save For Later Submit Ass © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |arrow_forward
- How many signals do you expect in the H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red Note for advanced students: In this question, any multiplet is counted as one signal. 1 Number of signals in the 'H NMR spectrum. For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. Check For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. O ✓ No additional Hs to color in top molecule ง No additional Hs to color in bottom…arrow_forwardin the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstantarrow_forwardtrue or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


