
Concept explainers
Write the structure of the organic product in each of the following reactions. If electrophilic
aromatic substitution occurs, assume only monosubstitution.
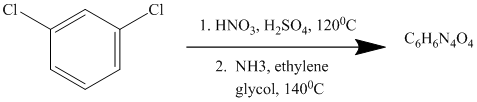
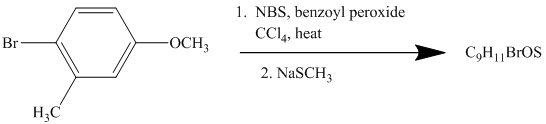
Interpretation:
Structures for the major organic product of an electrophilic aromatic monosubstituted reactions in each of the given reactions is to be written.
Concept introduction:
Ring activating groups such as hydroxide, amines, alkoxy groups etc. direct the incoming electrophile to ortho or para position with respect to their position.
Ring deactivating groups such as nitro, formyl, esters, acyl, etc. direct the incoming electrophile to meta position with respect to their position.
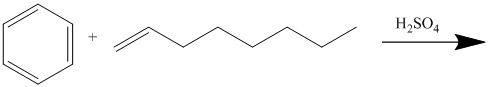
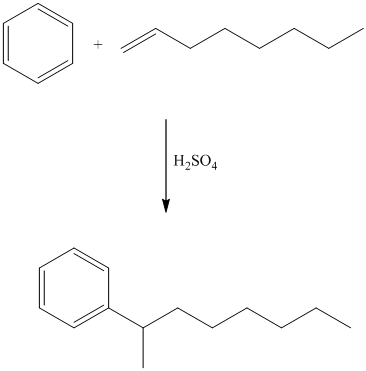
In electrophilic aromatic substitution, alkenes, which are converted to carbocations by protonation in the presence of a strong acid, can be used to alkylate benzene.
Aryl halides (halogens attached to the
Heterocyclic aromatic compounds such as pyrrole, furan, thiophene have electron rich aromatic rings and are extremely reactive towards electrophilic aromatic reactions. The incoming electrophile attaches selectively to C2 carbon atom in case of heterocyclic aromatic compounds.
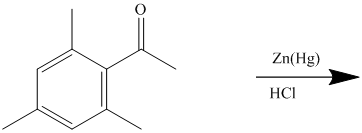
The reagent zinc amalgam and concentrated hydrochloric acid is used to convert a carbonyl group into methylene unit. This reaction is known as Clemmenson’s reduction.
Answer to Problem 33P
Solution:
Explanation of Solution
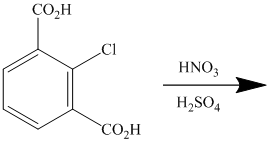
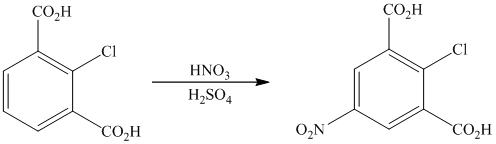
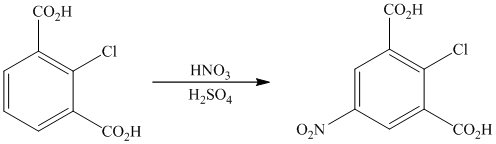
The given reactant molecule has a benzene ring with two carboxylic acid groups and one chlorine as substituents. The mixture of
The carboxylic acid groups are strong deactivating and meta directing groups. Chlorine atom slightly deactivates the ring but is ortho-para directing group. The incoming electrophile, nitronium ion, will be directed to the meta position with respect to the two carboxylic acid groups, which is also the para position with respect to the chlorine atom. Both the attached substituents reinforce electrophilic aromatic substitution at para position with respect to chlorine.
Thus, the reaction is as follows:
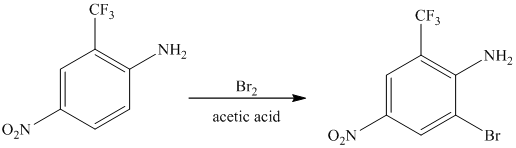
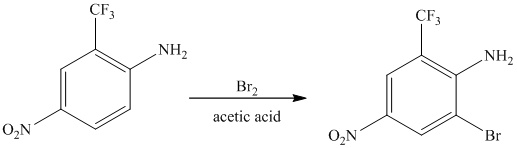
The given reactant molecule has a benzene ring attached to three substituents. The three substituents are amino group
Due to the presence of two strong deactiving substituents, the incoming electrophile is directed to the meta position with respect to
Thus, the reaction is as follows:
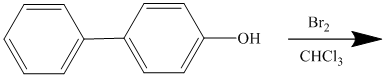
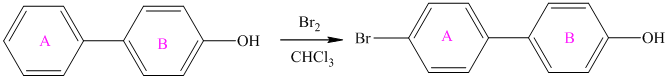
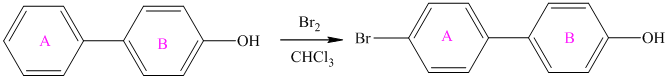
For a biphenyl compound, two benzene rings are connected to each other by a single bond. If only one substituent is present in the biphenyl ring, then its position is designated as being ortho, meta, or para with respect to the other ring.
In biphenyl, ring B is considered as a substituent of ring A. Ring B is an aryl ring with a hydroxyl group attached to it. Hydroxyl group is an activating group and is ortho-para directing. Thus, monosubstitution takes place to para position with respect to ring B.
Thus, the reaction is as follows:
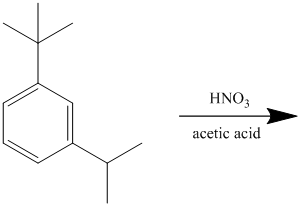
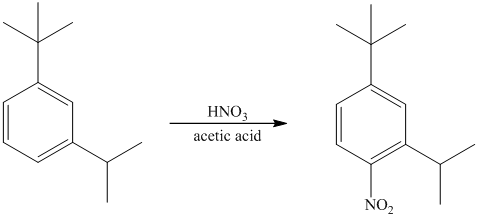
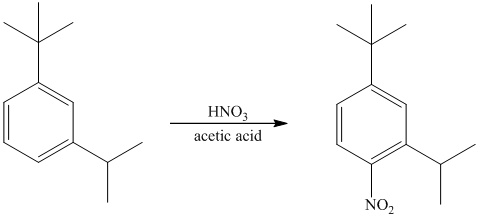
The given reactant molecule has a benzene ring attached to alkyl substituents. The one substituent is an isopropyl group while the other is tert-butyl group.
Both the substituents are strong activators and are ortho-para directors. Tert-butyl substituent is more sterically hindered than an isopropyl substituent. Thus, the electrophilic aromatic substitution takes place at ortho position with respect to the isopropy group, which is also the para position of tert-butyl group.
Thus, the reaction is as follows:
In the given reaction,
Thus, the reaction is as follows:
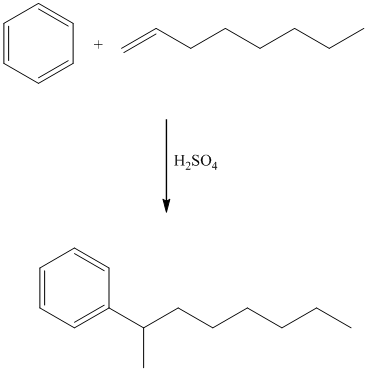
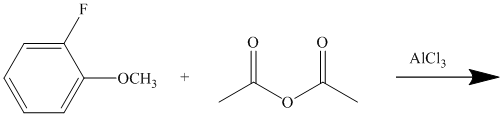
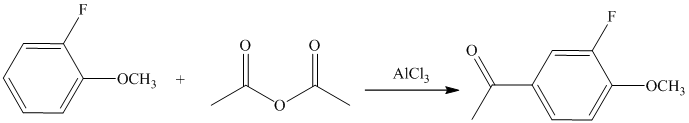
This is a Friedel Crafts acylation reaction. The benzene ring has two substituents attached –fluorine and methoxy groups. Acetic acid and aluminum chloride produce an acyl cation, which is an electrophile in this reaction.
The fluorine is a deactivator but ortho-para director. The methoxy group is a strong activator and ortho-para director. Thus, the strong activating methoxy group will direct the incoming acyl cation to its para position.
Thus, the reaction is as follows:
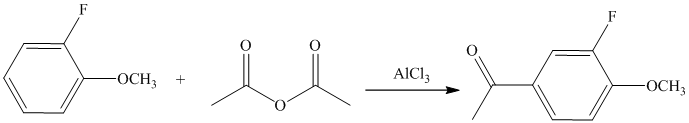
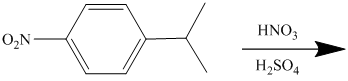
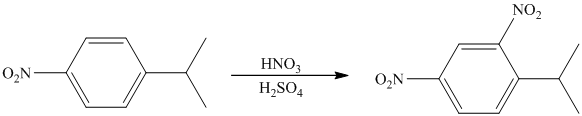
The given electrophilic aromatic substation reaction is nitration. The mixture of
The isopropyl group attached to the benzene ring is an activator and ortho-para director. The nitro group is a strong deactivator and meta director. When mononitration of this molecule takes place, the incoming electrophile is directed meta to nitro group, which is the para position with respect to the isopropyl group.
Thus, the reaction is as follows:
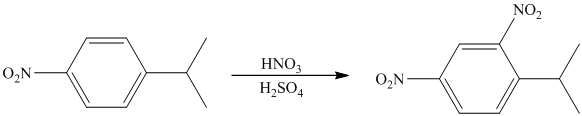
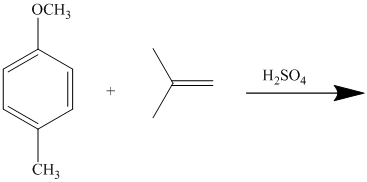
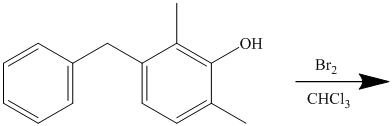
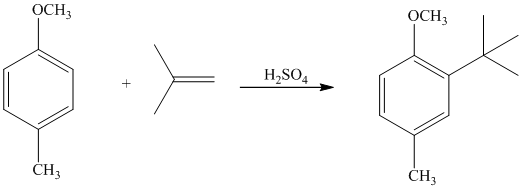
In the given reactant molecule, a benzene ring has two substituents attached. One substituent is the methoxy group while the other is methyl group. Both methyl and methoxy groups will activate the ring and are ortho-para directors.
The methoxy group activates the ring stronger than the methyl group. Thus, the alkylation takes place at ortho position with respect to methoxy group.
Thus, the reaction is as follows:
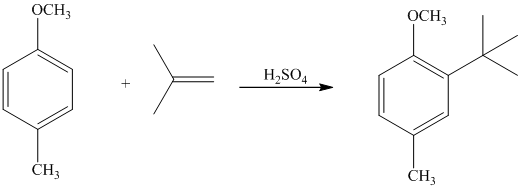
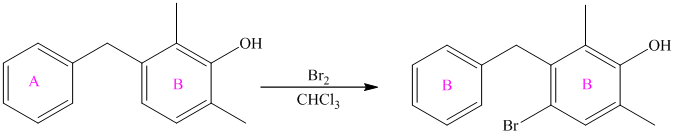
The given reactant molecule shows two benzene rings connected by a
When this compound undergoes electrophilic aromatic substitution, the incoming electrophile is directed to the para position with respect to the hydroxyl group in ring B, which is also the ortho position with respect to ring A.
The reaction is as follows:
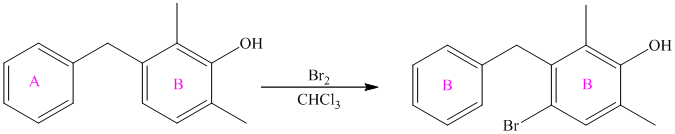
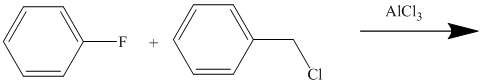
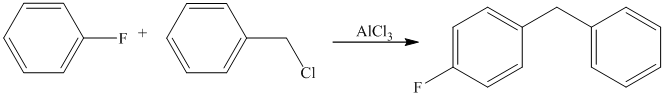
The given reactant molecule has a benzene ring attached to one fluorine while the other reactant is an benzyl chloride.
The fluoro group is a deactivator, but it will direct the aryl group in the para position. In this Friedel-Craft alkylation reaction, the benzyl group will be directed at the para position with respect to the fluorine atom.
Thus, the reaction is as follows:
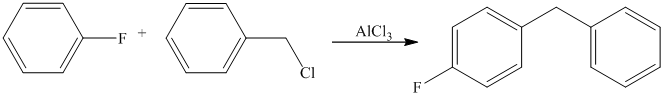
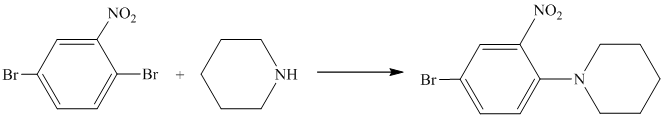
Aryl halides (halogens attached to the
Thus, the reaction is as follows:
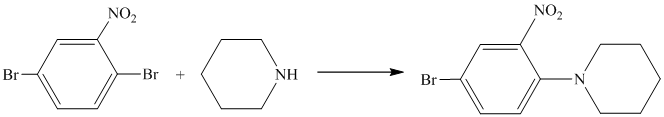
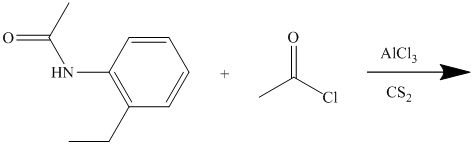
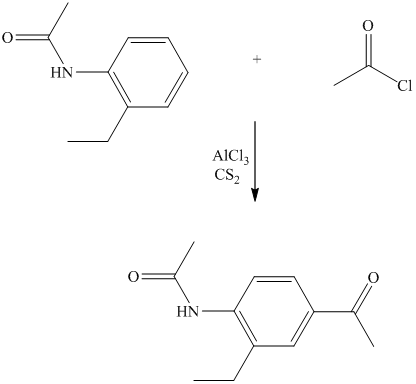
In the reactant molecule, a benzene ring is attached to two substituents. One substituent is acylamino while the other is ethyl substituent. Both these substituents are strong activators and ortho-para directors. This is a Friedel Crafts acylation reaction, in which the acyl group will add to the para position with respect to the acylamino group and ethyl group, which are strong activators.
Thus, the reaction is as follows:
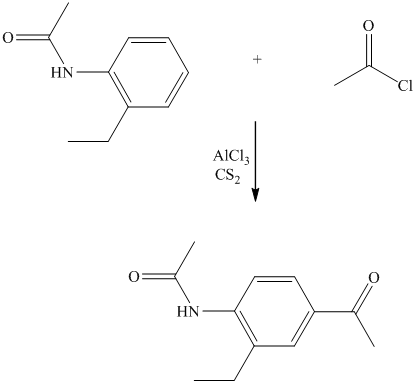
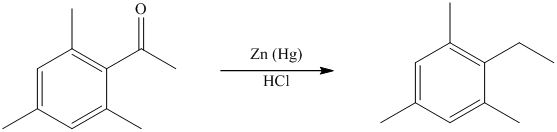
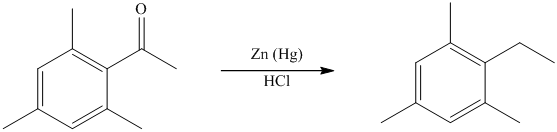
The give reactant benzene ring has four substituents. Three substituents are methyl groups while one is an acyl group. The reagent zinc amalgam and concentrated hydrochloric acid is used to convert a carbonyl group into methylene unit. This reaction is known as Clemmenson reduction.
Thus, the reaction is as follows:
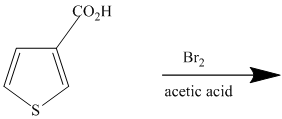
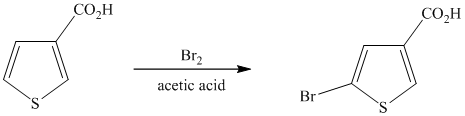
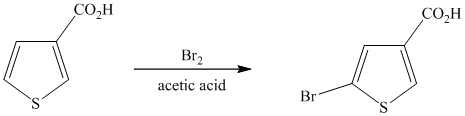
This is an example of a heterocyclic electrophilic aromatic substitution reaction. Thiophenes have electron rich aromatic rings and are extremely reactive towards electrophilic aromatic substitution, preferably at C2-C5 carbon atom in the ring. The thiophene ring has a carboxylic acid group as a substituent attached. Carboxylic acid group deactivates the ring and is a meta directing group. Thus, in bromination of substituted thiophene, the bromine will add to meta position with respect to the carboxylic acid group, which is also the C2 position of thiophene.
Thus, the reaction is as follows:
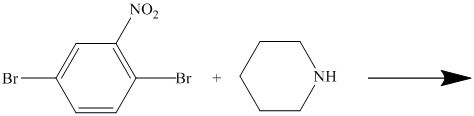
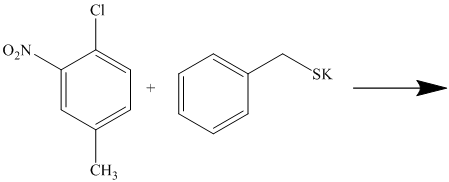
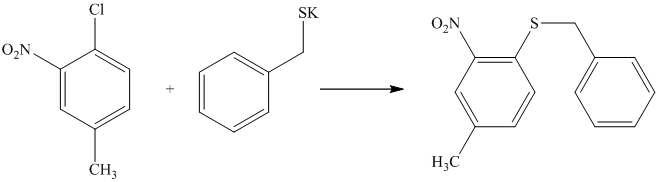
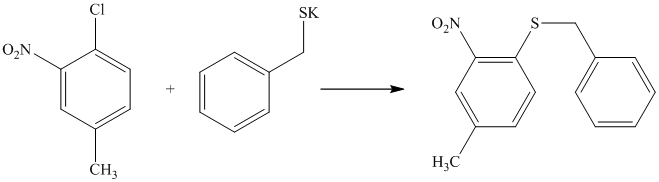
There is a strong deactivator group (nitro group) present at ortho position to the chlorine. Because of this, that chlorine will undergo nucleophilic substitution reaction. The nucleophile is sulfur, which will attack the chlorine and form the product.
Thus, the reaction is as follows:
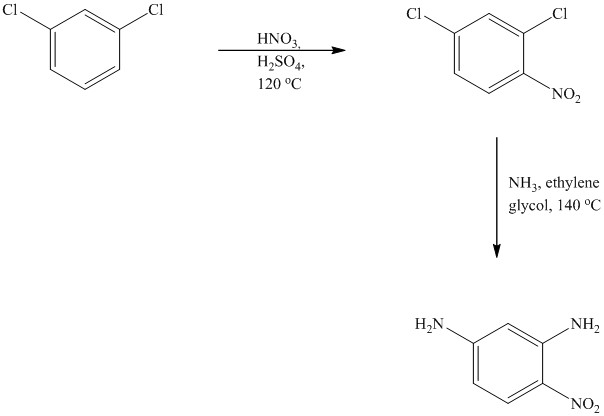
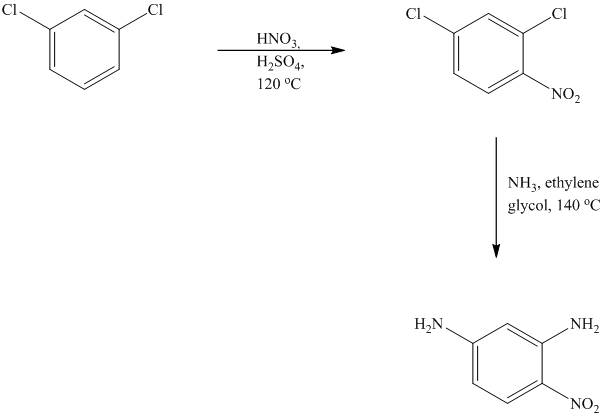
This is an example of an electrophilic aromatic substitution followed by a nucleophilic aromatic substitution. In the given reactant molecule, a benzene ring has two chloro groups, which are deactivators but ortho-para directors. The mixture of
As a first step, nitration of the given reactant molecule takes place such that the nitro group will be attached to the ortho position with respect to one of the chlorine atoms and para with respect to the other chlorine atom. Thus, in the product for this step one, there is a benzene ring with three substituents, the two chlorine atoms meta to each other and one nitro group para to one chlorine and ortho to the other.
In this product, the nitro group is placed where it is ortho to one chlorine and para to the other one. Since there is a strong deactivator group (nitro group) present at ortho and para positions to both the chlorines, the aryl chloride will undergo nucleophilic substitution reaction. The nucleophile is ammonia. Both the chlorine atoms will be replaced by amino groups.
Thus, the reaction is as follows:
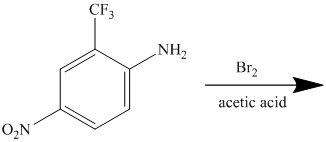
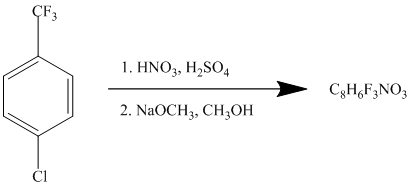
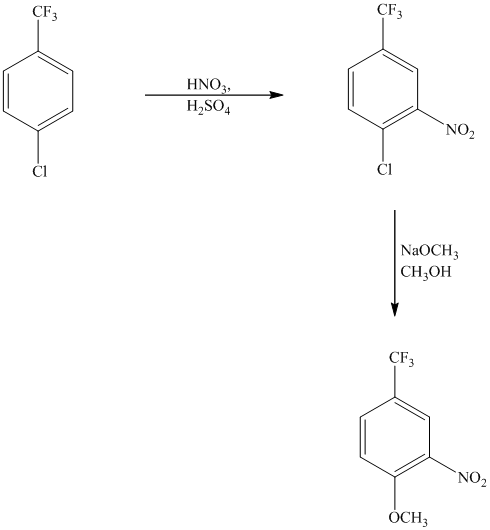
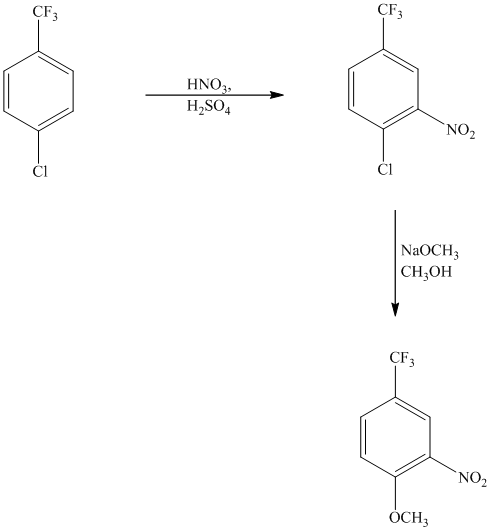
This is an example of an electrophilic aromatic substitution followed by a nucleophilic aromatic substitution. The mixture of
If the reactant molecule undergoes a nitration reaction, then the nitro group is placed ortho with respect to the chlorine atom, which is also the meta position with respect to the trifluoromethyl group.
This first step produces a compound in which two strong deactivating groups are attached at ortho and para positions with respect to the chlorine atom. Thus, a nucleophilic aromatic substitution reaction will take place. The nucleophile is methoxide, which will attack the chlorine and form the product.
Thus, the reaction is as follows:
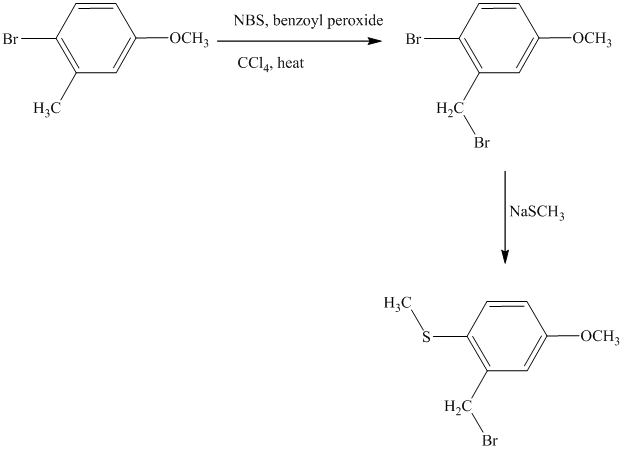
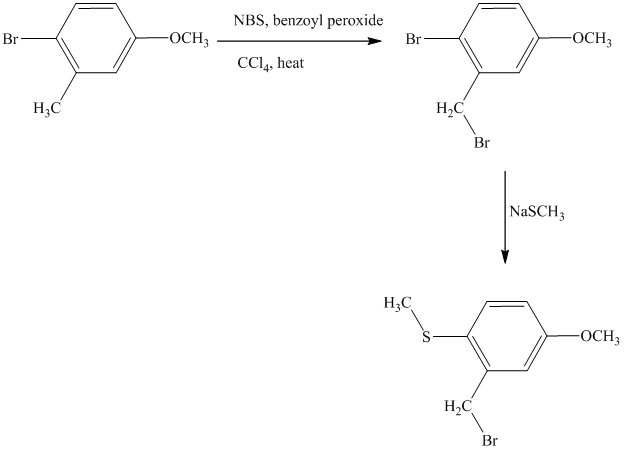
This is an example of an electrophilic aromatic substitution followed by a nucleophilic aromatic substitution. Here, NBS reagent adds bromine to benzylic carbon, so bromine will be added to the
Thus, the reaction is as follows:
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry - Standalone book
- Predict the products of this organic reaction: O N IN A N + H2O + HCI ? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. 田 C + Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward6. For each of the following, fill in the synthesis arrows with reagents and show the intermediates. You DO NOT need to use the same number of arrows that are shown (you may use more or less), but the product must be formed from the reactant. Then write the mechanism of one step in the synthesis (you can choose which step to write the mechanism for), including all reagents required, clearly labeling the nucleophile and electrophile for each step, and using curved arrows to show the steps in the mechanism. a. b. OHarrow_forwardDraw the productsarrow_forward
- Draw the correct productsarrow_forwardE Organic Chemistry Maxwell Draw the correct products, in either order, for the ozonolysis reaction: 1) O3, CH2Cl2, -78 °C Product 1 + Product 2 2) Zn, HOAc Draw product 1. Select Draw Templates More C H O presented by M Draw product 2. Erase Select Draw Templates M / # # carrow_forward✓ edict the products of this organic reaction: ---- ။ A CH3–C−NH–CH2–C−CH3 + KOH ? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibiliarrow_forward
- Predict the product of this organic reaction: A HO-C-CH3 + CH3NH2 P+ H2O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. marrow_forwardH 1) OsO4, pyridine 2) Na2SO3 or NaHSO3 in H₂O 2 productsarrow_forward● Biological Macromolecules Naming and drawing cyclic monosaccharides Your answer is incorrect. • Row 1: Your answer is incorrect. Row 3: Your answer is incorrect. • Row 4: Your answer is incorrect. Try again... 0/5 Give the complete common name, including anomer and stereochemistry labels, of the following molecules. You will find helpful information in the ALEKS resource. CH2OH OH OH H H I H OH OH H] H CH2OH H OH ẞ-L-sorbose HOCH2 OH OH H HOCH2 H OH OH H OH H H CH2OH OH H H OH H I- H OH H OH Explanation Recheck W E R % 25 α B Y X & 5 D F G H McGraw Hill LLC. All Rights Reserved. Terms of Use | Pr Parrow_forward
- What is the missing reactant in this organic reaction? + R -A HO IN + H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardStuc X ctclix ALE X A ALE אן A ALEX Lab (195 X Nut x M Inb x NU X NUT X Unt x + → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpZuyrVCdQolLAKqp_7U3r1GUD3... New Chrome available: Naomi Question 26 of 39 (4 points) | Question Attempt: 1 of Unlimited Give the IUPAC name. 2,3-dimethylhexane Part: 1/2 Part 2 of 2 Draw the skeletal structure of a constitutional isomer of the alkane above that contains a different number of carbons in its longest chain. Skip Part Check Click and drag to start drawing a structure. 3 Finance headline Q Search mwa Harvard Intensifi... X Save For Later 00 dlo HB Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility a 9:11 PM 4/22/2025arrow_forwardPredict the product of this organic reaction: + NH2 HO A P+ H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Click and drag to start drawing a structure. ✓arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



