
Bundle: Chemistry for Today: General, Organic, and Biochemistry, Loose-Leaf Version, 9th + LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card
9th Edition
ISBN: 9781337598255
Author: Spencer L. Seager
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
thumb_up100%
Chapter 13, Problem 13.53E
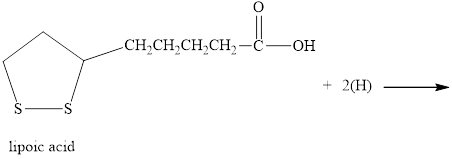
Lipoic acid is required by many microorganisms for proper growth. As a disulfide, it functions in the living system by catalyzing certain oxidation reactions and is reduced in the process. Write the structure of the reduction product.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Questions 4 and 5
For a titration of 40.00 mL of 0.0500 M oxalic acid H2C2O4 with 0.1000 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin;2) 15 mL; 3) 20 mL; 4) 25 mL; 5) 40 mL; 6) 50 mL. Ka1 = 5.90×10^-2, Ka2 = 6.50×10^-5 for oxalic acid.
Predict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.
Chapter 13 Solutions
Bundle: Chemistry for Today: General, Organic, and Biochemistry, Loose-Leaf Version, 9th + LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card
Ch. 13 - Draw general formulas for an alcohol and phenol,...Ch. 13 - Prob. 13.2ECh. 13 - Assign IUPAC names to the following alcohols: a....Ch. 13 - Assign IUPAC names to the following alcohols: a....Ch. 13 - Several important alcohols are well known by...Ch. 13 - Prob. 13.6ECh. 13 - Draw structural formulas for each of the...Ch. 13 - Draw structural formulas for each of the...Ch. 13 - Name each of the following as a derivative of...Ch. 13 - Name each of the following as a derivative of...
Ch. 13 - Prob. 13.11ECh. 13 - Draw structural formulas for each of the...Ch. 13 - Prob. 13.13ECh. 13 - Classify the following alcohols as primary,...Ch. 13 - Classify the following alcohols as primary,...Ch. 13 - Draw structural formulas for the four aliphatic...Ch. 13 - Why are the boiling points of alcohols much higher...Ch. 13 - Arrange the compounds of each group in order of...Ch. 13 - Prob. 13.19ECh. 13 - Draw structural formulas for the following...Ch. 13 - Prob. 13.21ECh. 13 - Draw the structures of the chief product formed...Ch. 13 - Draw the structures of the chief product formed...Ch. 13 - Draw the structures of the ethers that can be...Ch. 13 - Prob. 13.25ECh. 13 - Give the structure of an alcohol that could be...Ch. 13 - Give the structure of an alcohol that could be...Ch. 13 - What products would result from the following...Ch. 13 - What products would result from the following...Ch. 13 - Each of the following conversions requires more...Ch. 13 - Each of the following conversions requires more...Ch. 13 - The three-carbon diol used in antifreeze is It is...Ch. 13 - Methanol is fairly volatile and evaporates quickly...Ch. 13 - Prob. 13.34ECh. 13 - Prob. 13.35ECh. 13 - Name an alcohol used in each of the following...Ch. 13 - Prob. 13.37ECh. 13 - Prob. 13.38ECh. 13 - Assign a common name to each of the following...Ch. 13 - Assign a common name to each of the following...Ch. 13 - Assign the IUPAC name to each of the following...Ch. 13 - Assign the IUPAC name to each of the following...Ch. 13 - Prob. 13.43ECh. 13 - Draw structural formulas for the following: a....Ch. 13 - Prob. 13.45ECh. 13 - Prob. 13.46ECh. 13 - Prob. 13.47ECh. 13 - Arrange the following compounds in order of...Ch. 13 - Arrange the compounds in Exercise 13.48 in order...Ch. 13 - Prob. 13.50ECh. 13 - Complete the following reactions: a. b....Ch. 13 - Prob. 13.52ECh. 13 - Lipoic acid is required by many microorganisms for...Ch. 13 - Alcohols and thiols can both be oxidized in a...Ch. 13 - Prob. 13.55ECh. 13 - Prob. 13.56ECh. 13 - Prob. 13.57ECh. 13 - Thiols have lower boiling points and are less...Ch. 13 - Prob. 13.59ECh. 13 - Prob. 13.60ECh. 13 - Prob. 13.61ECh. 13 - Prob. 13.62ECh. 13 - A mixture of ethanol and 1propanol is heated to...Ch. 13 - Prob. 13.64ECh. 13 - Prob. 13.65ECh. 13 - Prob. 13.66ECh. 13 - Prob. 13.67ECh. 13 - Figure 13.8 points out that methanol is used as a...Ch. 13 - Figure 13.13 focuses on the use of thiol chemistry...Ch. 13 - Prob. 13.70ECh. 13 - Prob. 13.71ECh. 13 - Prob. 13.72ECh. 13 - The compound that has the greatest polarity is: a....Ch. 13 - Alcoholic beverages contain: a. wood alcohol. b....Ch. 13 - Prob. 13.75ECh. 13 - Which of the following compounds is an ether? a....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.arrow_forwardHow many signals would you expect to find in the 1 H NMR spectrum of each given compound? Part 1 of 2 2 Part 2 of 2 HO 5 ☑ Х IIIIII***** §arrow_forwardA carbonyl compound has a molecular ion with a m/z of 86. The mass spectra of this compound also has a base peak with a m/z of 57. Draw the correct structure of this molecule. Drawingarrow_forward
- Can you draw this using Lewis dot structures and full structures in the same way they are so that I can better visualize them and then determine resonance?arrow_forwardSynthesize the following compound from cyclohexanol, ethanol, and any other needed reagentsarrow_forwardFor a titration of 20.00 mL of 0.0500 M H2SO4 with 0.100 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin; 2) 10.00 mL; 3) 20.00 mL; 4) 30.00 mL. Ka2 = 1.20×10-2 for H2SO4.arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s) Be sure to account for all bond-breaking and bond-making steps Problem 73 of 10 Drawing Amows ro HO Donearrow_forward12. Synthesize the following target molecules (TMs) using the specified starting materials. .CI a) HO3S SM TM b) HO- SMarrow_forwardFor a titration of 20.00 mL of 0.0500 M H2SO4 with 0.100 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin; 2) 10.00 mL; 3) 20.00 mL; 4) 30.00 mL. Ka2 = 1.20×10-2 for H2SO4.arrow_forward
- Write the systematic name of each organic molecule: structure name show work. don't give Ai generated solutionarrow_forwardShow work with explanation needed. Don't give Ai generated solutionarrow_forwardA Elschboard Part of SpeechT-D Alt Leaming App app.aktiv.com Curved arrows are used to illustrate the flow of electrons. Using the provided resonance structures, draw the curved electron- pushing arrows to show the interconversion between resonance hybrid contributors. Be sure to account for all bond-breaking and bond-making steps. Include all lone pairs and formal charges in the structures. Problem 45 of 10 I Select to Add Arrows N Please selarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY