
What products would result from the following processes?
Write an equation for each reaction.
a.
b.
c.
d.
e.
(a)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.29E
The product formed when
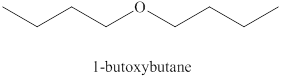
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Alcohols on heating at
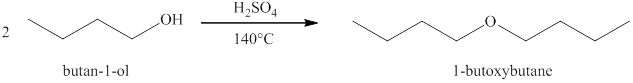
Figure 1
The product formed when
(b)
Interpretation:
The product formed by the excess oxidation of
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.29E
The product formed by the excess oxidation of
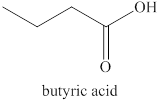
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
The primary alcohol,

Figure 2
The product formed by the excess oxidation of
(c)
Interpretation:
The product formed by the controlled oxidation of
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.29E
The product formed when
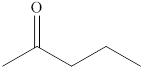
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
The compound
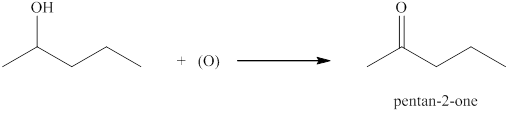
Figure 3
The product formed when
(d)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.29E
The product formed when
![]()
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Alcohols on heating at temperature
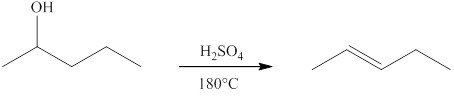
Figure 4
Interpretation:
The product formed when
(e)
Interpretation:
The product formed by the controlled oxidation of
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.29E
No product is formed by the controlled oxidation of
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
The controlled oxidation of
No product is formed by the controlled oxidation of
Want to see more full solutions like this?
Chapter 13 Solutions
Bundle: Chemistry for Today: General, Organic, and Biochemistry, Loose-Leaf Version, 9th + LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





