
Bundle: Chemistry for Today: General, Organic, and Biochemistry, Loose-Leaf Version, 9th + LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card
9th Edition
ISBN: 9781337598255
Author: Spencer L. Seager
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.15E
Classify the following alcohols as primary, secondary, or tertiary:
a. 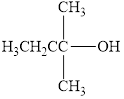
b.
c. 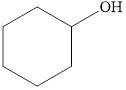
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
61. The symbol shown below is which of the follow ing?
H.
C-OH
a structural formula
a stick formula
an empir ical formula
a.
с.
b.
a molecular formula
d.
Ethano ic acid (vinegar) when diluted to low concentrations by water can be prepared from ethene by
reduction with H2, followed by reaction with a strong oxidizer
b. addition of HCI, followed by reaction with H,O
addition of H,O followed by reaction with a strong oxidizer
d.
62.
а.
с.
addition of Br,, followed by reduction with H2
63. A cosmetic company wants to produce nail polish remover, also known as acetone,
H3C-C
-CH3,
An efficient method would involve the follow ing series of reactions in which order?
from
propene.
halogenation, oxidation
b. reduction, hydration
a.
c. reduction, ha logenation, elimination
d. hydration, oxidation
7.
H3C-CH
The compound above is classified as a(n)
alkane
d. ketone
alkene
a.
e.
b. carboxylic acid
c. akdehyde
8.
Which of the follow ing is a secondary alcohol?
d. CH3OH
a.
H3C C
CH3
CH3
b. H3C0-CH3
e. CH3CH2OH
c.
OH
H3C
CH3
H.
9.
CH3 OH
-CH-CH-CH,
H3C
What is the correct name for the above compound?
a. 2-methyl-3-butanol
b. 2-pentanol
isobutanol
d. 3-methy-2-butanol
none of these
e.
c.
___ is an example of an alkyl halide.
Select one:
a. KCl
b. CHCl3
c. NaCl
d. CF2=CF2
Chapter 13 Solutions
Bundle: Chemistry for Today: General, Organic, and Biochemistry, Loose-Leaf Version, 9th + LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card
Ch. 13 - Draw general formulas for an alcohol and phenol,...Ch. 13 - Prob. 13.2ECh. 13 - Assign IUPAC names to the following alcohols: a....Ch. 13 - Assign IUPAC names to the following alcohols: a....Ch. 13 - Several important alcohols are well known by...Ch. 13 - Prob. 13.6ECh. 13 - Draw structural formulas for each of the...Ch. 13 - Draw structural formulas for each of the...Ch. 13 - Name each of the following as a derivative of...Ch. 13 - Name each of the following as a derivative of...
Ch. 13 - Prob. 13.11ECh. 13 - Draw structural formulas for each of the...Ch. 13 - Prob. 13.13ECh. 13 - Classify the following alcohols as primary,...Ch. 13 - Classify the following alcohols as primary,...Ch. 13 - Draw structural formulas for the four aliphatic...Ch. 13 - Why are the boiling points of alcohols much higher...Ch. 13 - Arrange the compounds of each group in order of...Ch. 13 - Prob. 13.19ECh. 13 - Draw structural formulas for the following...Ch. 13 - Prob. 13.21ECh. 13 - Draw the structures of the chief product formed...Ch. 13 - Draw the structures of the chief product formed...Ch. 13 - Draw the structures of the ethers that can be...Ch. 13 - Prob. 13.25ECh. 13 - Give the structure of an alcohol that could be...Ch. 13 - Give the structure of an alcohol that could be...Ch. 13 - What products would result from the following...Ch. 13 - What products would result from the following...Ch. 13 - Each of the following conversions requires more...Ch. 13 - Each of the following conversions requires more...Ch. 13 - The three-carbon diol used in antifreeze is It is...Ch. 13 - Methanol is fairly volatile and evaporates quickly...Ch. 13 - Prob. 13.34ECh. 13 - Prob. 13.35ECh. 13 - Name an alcohol used in each of the following...Ch. 13 - Prob. 13.37ECh. 13 - Prob. 13.38ECh. 13 - Assign a common name to each of the following...Ch. 13 - Assign a common name to each of the following...Ch. 13 - Assign the IUPAC name to each of the following...Ch. 13 - Assign the IUPAC name to each of the following...Ch. 13 - Prob. 13.43ECh. 13 - Draw structural formulas for the following: a....Ch. 13 - Prob. 13.45ECh. 13 - Prob. 13.46ECh. 13 - Prob. 13.47ECh. 13 - Arrange the following compounds in order of...Ch. 13 - Arrange the compounds in Exercise 13.48 in order...Ch. 13 - Prob. 13.50ECh. 13 - Complete the following reactions: a. b....Ch. 13 - Prob. 13.52ECh. 13 - Lipoic acid is required by many microorganisms for...Ch. 13 - Alcohols and thiols can both be oxidized in a...Ch. 13 - Prob. 13.55ECh. 13 - Prob. 13.56ECh. 13 - Prob. 13.57ECh. 13 - Thiols have lower boiling points and are less...Ch. 13 - Prob. 13.59ECh. 13 - Prob. 13.60ECh. 13 - Prob. 13.61ECh. 13 - Prob. 13.62ECh. 13 - A mixture of ethanol and 1propanol is heated to...Ch. 13 - Prob. 13.64ECh. 13 - Prob. 13.65ECh. 13 - Prob. 13.66ECh. 13 - Prob. 13.67ECh. 13 - Figure 13.8 points out that methanol is used as a...Ch. 13 - Figure 13.13 focuses on the use of thiol chemistry...Ch. 13 - Prob. 13.70ECh. 13 - Prob. 13.71ECh. 13 - Prob. 13.72ECh. 13 - The compound that has the greatest polarity is: a....Ch. 13 - Alcoholic beverages contain: a. wood alcohol. b....Ch. 13 - Prob. 13.75ECh. 13 - Which of the following compounds is an ether? a....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you please answer on #2 and #3? Thanksarrow_forward3. 1. Which of the following is an alcohol? a. NaOH b. H3C C. -NH₂ OH d. CH 3 CH 2 OH e. a. 1s²2s²2p¹3s2² b. 1s¹2s¹2p 3s² c. 1s²2s²2p6 H3C H3C 2. How many actual double bonds does the benzene ring possess? d. 1 e. 0 -CH3 a. 4 b. 3 C. 2 Which of the following is the electron configuration for neon? d. 1s³2s³2p4 e. 1s²2s²2p8arrow_forwardWhat is the organic product formed in the following reaction? CH3- I III H₂C. Select one: A. IV B. III C. II O D. I Br H H₂C. My H OH H II IV H₂C H₂C. HC H Harrow_forward
- 1. Identify the functional groups in the following molecules, and show the polarity of each: NH2 CH,CH,C=N b. a. .CH -OCH3 d. C. е.arrow_forwardA. Classification of Hydrocarbons. H H 6. H-C=C-C-C-H H H 2. H-C-Ċ-H H HHH H-C=C-C=C-H 8. CH CH; CH CH-CH,-CH, CH: CH, CH, CH, 10.arrow_forward4 H₂C H3C C=O + NaBH4 + 38 g/mol 58 g/mol 4 CH3OH A. 51.7% B. 78.9% C. 50% D. 25% E. 75% 4 H3C H3C OH H 60 g/mol Suppose that the reaction of 5.8 g of acetone with 3.8 g of NaBH4 gives 1.5 g of isopropyl alcohol. What is the % yield of isopropyl alcohol? Circle the correct answer. + NaB(OCH3)4arrow_forward
- 10. How many H-bonds can a secondary alcohol form? A. 3 B. 1 C. 2 D. 0arrow_forward1. What is the chemical formula for the following molecules a. an alkane with 9 carbons: b. an alkene with 5 carbons (assume 1 double bond): C. an alkyne with 8 carbons (assume 1 triple bond):arrow_forward2.24 g of alkene A add 3.2 g of bromine. The molecular formula of A is: A. C10H20 B. C2H4 C. C6H12 D. C8H16arrow_forward
- How many moles of bonds between which pairs of atoms are broken during the combustion of 3 moles of methane (CH4) gas? Treat a double bond or a triple bond as one bonding interaction (i.e., 1 mole of triple bonds equals 1 mole of bonds). Choose one or more: A. 12 moles O–H bonds B. 6 moles of C–O bonds C. 3 moles of C–O bonds D. 6 moles of O–H bonds E. 3 moles of C–H bonds F. 6 moles of O–O bonds G. 12 moles of C–H bondsarrow_forwardB. Θ (CH3)3NH CIO D. H3CC=CCH₂OHarrow_forwardCategorize the chemical structure shown here CH3 CH3 H-CH CH-C-CH3 A. Alkane B. Alkyne C. Alkene D. Cycloalkanearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License