
Concept explainers
The equilibrium constant for the conjugate acid-base pair
(a) calculate the absorbance at 430 nmand 600 nm for the following indicator concentrations:
3.00 × 10-4M,2.00 × 10-4M, 1.00 × 10-4M, 0.500 × 10-4 M, and 0.250 × 10-4M.
(b) plot absorbance as a function of indicator concentration.

(a)
Interpretation:
Absorbance values of the conjugate acid-base pair solutions of different concentrations at
Concept introduction:
The absorbance can be calculated using the following formula:
For the given conjugate acid-base pair, equilibrium can be written as,
Answer to Problem 13.11QAP
Explanation of Solution
Let’s use the equilibrium equation to find the concentrations of HIn and In-(denoted as [HIn] and [In-]) at a situation in which the total concentration (cHIn) is
Now, the absorbance values of this solution at 430 and 600 nm can be calculated as follows,
The cell length (b) is not given in the question, therefore taken as 1.00cm.
Similarly, other absorbance values can be calculated for solutions with different concentrations.
(b)
Interpretation:
Absorbance as a function of indicator concentration should be plotted.
Explanation of Solution
The data obtained is as follows:
From the data, the graph between absorbance and concentration can be plotted as follows:
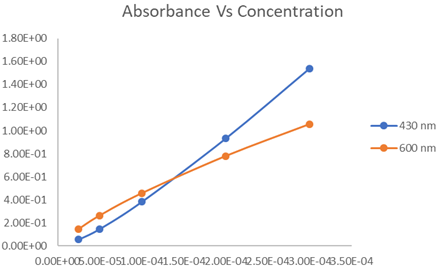
Here, blue curve indicates the plot at 430 nm and orange curve indicates the same at 600 nm.
Want to see more full solutions like this?
Chapter 13 Solutions
PRINCIPLES OF INSTRUMENTAL ANALYSIS
- Write the chemical equation and the expression for the equilibrium constant, and calculate Kb for the reaction of each of the following ions as a base. (a) sulfate ion (b) citrate ionarrow_forwardEquilibrium constants can be measured for the dissociation of Lewis acid-base complexes such as the dimethyl ether complex of BF3, (CH3)20→BF3. The value of K (here K,) for the reaction is 0.17 at 125 PC. (CH3)20 BF3 (g) 2 BF, (g) + (CHs)20(g) If 1.3 g of the complex is placed in a 75.5 mL flask at 125 degrees Celsius, what would the partial pressures (in atm) of the Lewis acid, Lewis base, and complex be? What would the total pressure (in atm) in the flask at equilibrium be?arrow_forwardCalculate the equilibrium concentration of HC2O4−HC2O4− in a 0.20 mol L−1mol L−1 solution of oxalic acid. Express your answer to two significant figures and include the appropriate unitsarrow_forward
- Calculate the pH at 25 °C of a 0.53M solution of ethylammonium bromide (C,H,NH, Br). Note that ethylamine (C,H,NH,) is a weak base with a p K, of 3.19. Round your answer to 1 decimal place. pH = [|arrow_forwardCalculate the pH of the following solutions at 298K: (a) potassium hydroxide 2.4 x 104 mol dm3 (b) nitric acid 0.0078 mol dm3 5cm of 1.0 mol dm3 H2SO4 that is diluted to 250cm3 with distilled water. Assume the sulfuric acid Is completely dissociated. (c) Kwi208) = 1 x 10-14 mol? dm6arrow_forwardCOHSOH(ag) + H2On + CeHsO (aq) + H3O*(a9) Ka= 1.12 x 10-10 (a) Phenol is a weak acid that partially dissociates in water according to the equation above. Write the equilibrium-constant expression for the dissociation of the acid in water. (b) What is the pH of a 0.75 M CaHsOH(ag) solution? (C) For a certain reaction involving CaHsOH(ag) to proceed at a significant rate, the phenol must be primarily in its deprotonated form, C3H5O (eg). In order to ensure that the CsHsOH(aq) is deprotonated, the reaction must be conducted in a buffered solution. On the number scale below, circle each pH for which more than 50 percent of the phenol molecules are in the deprotonated form (CoHsO (aq). Justify your answer. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Justification: (d) CeHsOH(ag) reacts with NaOH(ag). Write a net ionic equation representing this reaction (aka: invasion equation). (e) What is the pH of the resulting solution when 30 mL of 0.40 M CSH5OH(aq) is added to 25 mL of 0.60 M NAOH. Show all work…arrow_forward
- Arrange the following solution from lowest pH to highest pH: 0.75 М CНH,COОН (К, 3 1.8 х 10-5) 0.01 М HNOZ (К, — 4.6 х 10-4) 0.05 М СНООН (К, — 1.8 х 10-4) %3D 0.75 М CH3СООН < 0.01 М HNOZ < 0.0.05 М СНООН b. 0.75 M СH,COОH < 0.05 М СНООН < 0.01 М HNOZ 0.01 М HNO, < 0.05 М СНООН <0.75 М СН,СООН d. 0.05 M СНООН < 0.01 М HNOZ <0.75 М СН,СООН а. С.arrow_forwardIn the laboratory, a general chemistry student measured the pH of a 0.371 M aqueous solution of acetylsalicylic acid (aspirin), HC,H-04 to be 1.991. Use the information she obtained to determine the Ka for this acid. K(experiment) =arrow_forwardplease answer all questionsarrow_forward
- A diprotic acid, H, A, has acid dissociation constants of K,j = 1.45 x 10-4 and K2 = 5.12 x 10-". Calculate the pH and molar concentrations of H,A, HA, and A²- at equilibrium for each of the solutions. A 0.184 M solution of H,A. pH = 2.2865 [H,A] = 0.1788 [HA"] = 5.17 xI10-3 [A?-] = M 5.12 x10-11 M A 0.184 M solution of NaHA. pH= [H,A] = M. [HA-] = [A2-] = M Marrow_forward9) The dissociation equilibrium constants for the protonated form of alanine (a diprotic amino acid, H₂X+) are Kal = 4.6 × 10-3 and Ka2=2.0 × 10-10. What is the pH of 50.00 mL of a 0.050 M solution of alanine after 37.50 mL of 0.100 M NaOH has been added? Para Ofind molesarrow_forwardThe pH of a 0.105 M HAT solution, where HA is the intermediate form of a diprotic acid with pK₁ = 3.56 and pK₂ = 7.82, is 3.56 O 7.82 O 5.69 1.78 3.91arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


